Global Gmp Cytokines Market
Market Size in USD Million
CAGR :
% 
 USD
904.38 Million
USD
1.65 Million
2024
2032
USD
904.38 Million
USD
1.65 Million
2024
2032
| 2025 –2032 | |
| USD 904.38 Million | |
| USD 1.65 Million | |
|
|
|
|
GMP Cytokines Market Size
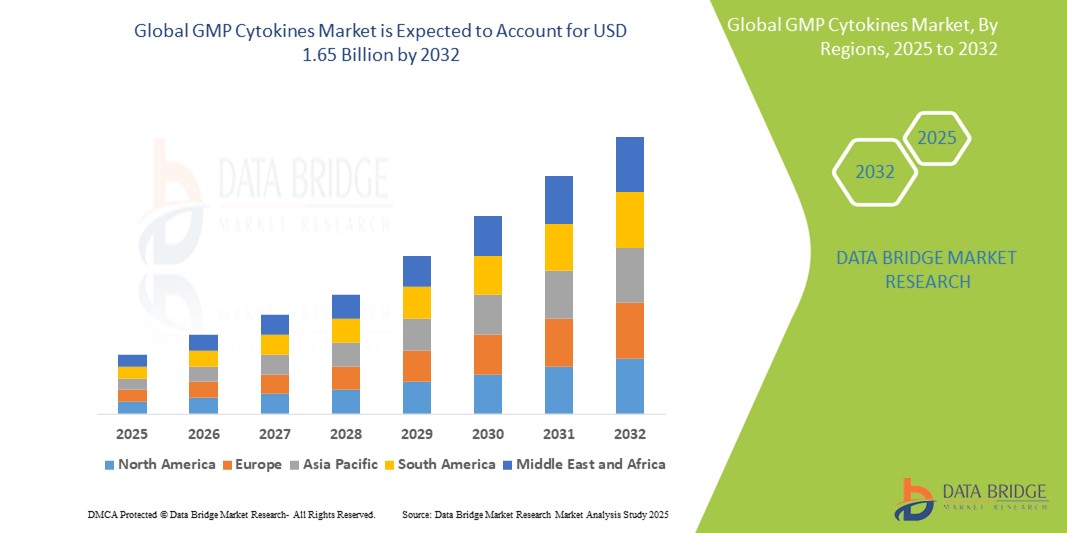
- The global GMP Cytokines Market was valued at USD 904.38 million in 2024 and is expected to reach USD 1.65 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 9.10%, primarily driven by the rising incidence of chronic diseases and autoimmune disorders
- This growth is driven by factors such as cancer immunotherapy, growing focus on personalized medicine, and advancements in technology
GMP Cytokines Market Analysis
- GMP Cytokines are critical components in advanced therapeutic applications, particularly in immunotherapy, cancer treatment, and regenerative medicine. They are essential for modulating immune responses and promoting cell growth, and are used in various medical procedures such as cancer immunotherapy, tissue regeneration, and autoimmune treatments
- The demand for GMP Cytokines is significantly driven by advancements in biotechnology and the increasing prevalence of chronic diseases, including cancer, autoimmune disorders, and inflammatory conditions. The global need for targeted therapies and personalized medicine has also contributed to the rising demand for GMP-grade cytokines, as they are crucial for ensuring the effectiveness and safety of these therapies
- North America stands out as one of the dominant markets for GMP Cytokines, driven by its strong pharmaceutical and biotech industries, advanced healthcare infrastructure, and high levels of investment in research and development. The region also benefits from regulatory frameworks that support the production and use of high-quality GMP-grade cytokines
- For instance, the U.S. continues to be a leader in clinical trials and biopharmaceutical development, with many biotech companies and research institutions utilizing GMP cytokines in their innovative therapies, particularly in oncology and immunology
- Globally, GMP Cytokines are recognized as an essential component in therapeutic development and are crucial for the success of modern cell-based therapies. They play a pivotal role in ensuring the efficacy, safety, and precision of these advanced medical treatments, making them indispensable in the development of cutting-edge therapies across various medical fields
Report Scope and GMP Cytokines Market Segmentation
|
Attributes |
GMP Cytokines Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
GMP Cytokines Market Trends
Increasing Focus on Personalized Medicine and Targeted Therapies
- One prominent trend in the global GMP cytokines market is the increasing focus on personalized medicine and targeted therapies
- This trend is reshaping the way cytokines are being utilized, with a shift toward customized treatments that are tailored to individual patient needs based on their genetic profile and specific disease characteristics
- For instance, personalized cancer immunotherapy involves selecting cytokines that are best suited to enhance the immune system's response against the patient's specific cancer type. This approach has led to more precise and effective treatments, especially in oncology and autoimmune disorders
- The demand for GMP cytokines is expanding as healthcare providers increasingly rely on these tailored therapies to improve patient outcomes and reduce side effects, which is driving innovation in the cytokine production process
- This trend is revolutionizing the landscape of drug development and therapy, as more targeted and effective treatments are being made available, which, in turn, increases the demand for GMP-grade cytokines in clinical trials and therapeutic applications
GMP Cytokines Market Dynamics
Driver
Growing Need Due to Prevalence of Chronic Diseases
- The rising prevalence of chronic diseases such as cancer, autoimmune disorders, and inflammatory conditions is significantly contributing to the increased demand for GMP cytokines
- As the global population ages, the incidence of these conditions continues to grow, with older adults being more prone to diseases that require targeted therapies, such as cytokine-based treatments
- Cancer, in particular, is one of the leading causes of death worldwide, requiring innovative therapies such as immunotherapy, where GMP cytokines play a crucial role in modulating immune responses and enhancing the efficacy of treatments
- Ongoing advancements in biotechnology and regenerative medicine highlight the increasing need for high-quality GMP cytokines to support cutting-edge therapies, ensuring precision and efficacy in treating these chronic diseases
- As more patients seek treatment for cancer, autoimmune diseases, and inflammatory conditions, the demand for GMP cytokines rises, facilitating improved therapeutic outcomes and reducing treatment-associated risks
For instance
- In September 2024, according to a study published by the National Institutes of Health, the rising incidence of cancer, particularly among individuals aged 65 years and older, drives the increasing need for immunotherapies and cytokine-based treatments. This trend contributes to the growing demand for GMP cytokines in the oncology market
- In August 2023, a report by the World Health Organization highlighted the global burden of autoimmune diseases, with an increasing number of patients requiring personalized cytokine therapies, further boosting the demand for GMP-grade cytokines
- The rising prevalence of chronic diseases such as cancer, autoimmune disorders, and inflammation significantly drives the global GMP cytokines market, ensuring the availability of safe, effective treatments
Opportunity
Advancements in Cytokine-Based Immunotherapies and Cell Therapies
- The development of novel cytokine-based immunotherapies and cell therapies presents a significant opportunity for the GMP cytokines market
- GMP cytokines are essential for enhancing immune responses in cancer treatments, such as chimeric antigen receptor T-cell (CAR-T) therapies, as well as in regenerative medicine applications such as stem cell therapies
- The increasing use of cytokines to enhance the body’s immune system and aid in tissue regeneration drives innovation in cytokine-based therapies
For instance
- In January 2025, according to a report published in The Lancet Oncology, cytokines such as interleukins and interferons are gaining traction in the development of CAR-T therapies, significantly improving the therapeutic potential of immunotherapies in cancer treatment. The rise of these advanced therapies creates a growing demand for GMP cytokines
- In February 2024, according to an article in the Journal of Translational Medicine, cytokine-based approaches in regenerative medicine are playing a critical role in wound healing, tissue regeneration, and organ repair, thus driving the market's expansion into new therapeutic areas
- As the adoption of cytokine-based immunotherapies and regenerative medicine continues to increase, there is a growing opportunity for GMP cytokines to play a pivotal role in improving patient outcomes and advancing personalized medicine
Restraint/Challenge
High Production Costs and Regulatory Hurdles
- The high production costs associated with GMP cytokines are a significant challenge, particularly impacting their affordability and accessibility for smaller healthcare providers and research institutions
- The stringent regulatory requirements for producing GMP-grade cytokines, along with the cost of compliance, further escalate the overall costs, making it difficult for smaller facilities, particularly in developing regions, to invest in these advanced therapies
- The process of manufacturing GMP cytokines requires stringent quality control and validation measures to ensure the safety and efficacy of the final product, contributing to high production costs
For instance
- In November 2024, according to a report by the International Federation of Pharmaceutical Manufacturers & Associations, the high costs of GMP cytokine production, due to rigorous quality standards and regulatory requirements, are affecting the affordability of these therapies, especially in low-income regions
- Consequently, these financial and regulatory barriers can limit the accessibility and adoption of GMP cytokines in certain markets, hindering overall market growth and widening the gap in healthcare equity
GMP Cytokines Market Scope
The market is segmented on the basis of type and application.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Application |
|
GMP Cytokines Market Regional Analysis
North America is the Dominant Region in the GMP Cytokines Market
- North America holds a dominant position in the GMP cytokines market, driven by its advanced healthcare infrastructure, high adoption of cutting-edge medical technologies, and strong presence of key pharmaceutical and biotechnology companies
- U.S. accounts for a significant market share due to its increased demand for innovative immunotherapies, growing prevalence of chronic diseases such as cancer, autoimmune disorders, and inflammatory diseases, and ongoing advancements in biotechnology
- The availability of well-established reimbursement policies and increasing government and private sector investments in research & development further strengthen the market
- In addition, the high rate of adoption of personalized medicine and targeted therapies in the U.S. drives the demand for GMP cytokines, as they are essential in immunotherapy and regenerative medicine
- The region’s strong regulatory framework and established clinical trial ecosystem ensure a steady supply of GMP cytokines, fueling market growth in North America
Asia-Pacific is Projected to Register the Highest Growth Rate
- Asia-Pacific is expected to witness the highest growth rate in the GMP Cytokines market, driven by rapid improvements in healthcare infrastructure, increasing awareness about chronic diseases, and rising surgical and therapeutic volumes
- Countries such as China, India, and Japan are emerging as key markets due to their growing aging populations, which are more susceptible to conditions such as cancer, autoimmune disorders, and chronic inflammatory diseases
- Japan stands out with its advanced medical technology, highly trained healthcare professionals, and a strong focus on cutting-edge immunotherapies, making it a crucial market for GMP cytokines. The country's demand for high-precision therapeutic solutions continues to rise, especially in oncology and regenerative medicine
- China and India, with their large populations and rising healthcare needs, are seeing increased government initiatives and private sector investments in modern medical and biopharmaceutical setups. The expanding presence of global pharmaceutical companies and improving healthcare access across these nations further contribute to the region's rapid market growth
- The increasing prevalence of chronic diseases, along with the growing adoption of advanced therapeutic and immunotherapy solutions, is expected to drive the demand for GMP cytokines in the Asia-Pacific region, positioning it as a key area for market expansion
GMP Cytokines Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Miltenyi Biotec (Germany)
- Sino Biological, Inc. (China)
- ABCAM Limited (U.K.)
- PeproTech Inc. (U.S.)
- Akron Biotech (U.S.)
- Sartorius CellGenix GmbH (Germany)
- Bio-Techne (U.S.)
- Creative Bioarray (U.S.)
- Proteintech Group, Inc. (U.S.)
- REPROCELL Inc. (Japan)
- Sartorius CellGenix GmbH (Germany)
- ACROBiosystems (U.S.)
Latest Developments in Global GMP Cytokines Market
- In June 2019, CellGenix expanded its cytokine production facility with a new automated filling and freeze-drying line. This expansion addresses the increasing demand for GMP-grade cytokines and supports scalable production for clinical applications in cell and gene therapy
- In April 2020, Proteintech Group, Inc. received ISO 13485 certification for its HumanKine human cell-expressed cytokines and growth factors. The certification reinforces the company’s commitment to GMP standards in cytokine production, ensuring consistent quality and safety for clinical and therapeutic use
- In October 2020, Akron Biotech announced the acquisition of Cytiva to enhance its GMP-compliant manufacturing capabilities. The integration of Cytiva’s FlexFactory system aims to improve the supply of critical materials needed for advanced therapy manufacturing, including cell and gene therapies
- In March 2024, Synthekine Inc. initiated Phase 1a/1b clinical trials for STK-012, its α/β biased IL-2 partial agonist. The trials aim to evaluate the safety and efficacy of this novel cytokine therapeutic in treating cancer and autoimmune diseases. This marks a significant step forward in the development of engineered cytokine-based therapies for immuno-oncology
- In September 2024, Scale Ready and Bio-Techne Announce Optimal Closed System Cytokine Packaged for Single Step Use in Closed System G-Rex Manufacture of CAR-T and TCR Therapies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.