Global Heat Stable Vaccine Formulations Market
Market Size in USD Billion
CAGR :
% 
 USD
17.12 Billion
USD
43.10 Billion
2024
2032
USD
17.12 Billion
USD
43.10 Billion
2024
2032
| 2025 –2032 | |
| USD 17.12 Billion | |
| USD 43.10 Billion | |
|
|
|
|
Heat-Stable Vaccine Formulations Market Size
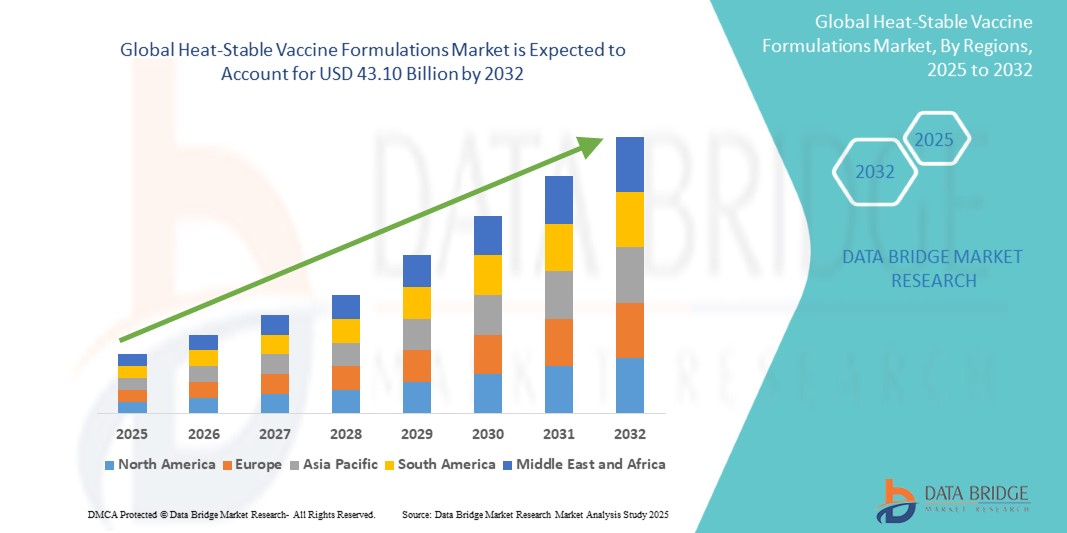
- The global heat-stable vaccine formulations market size was valued at USD 17.12 billion in 2024 and is expected to reach USD 43.10 billion by 2032, at a CAGR of 12.23% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress in heat-stable pharmaceutical formulations and cold-chain-free logistics, leading to increased accessibility of vaccines in both remote and resource-limited settings
- Furthermore, rising global demand for temperature-resilient, easy-to-administer, and transport-friendly vaccine solutions is establishing heat-stable vaccine formulations as the preferred alternative to traditional cold chain-dependent vaccines. These converging factors are accelerating the uptake of heat-stable vaccine formulations, thereby significantly boosting the industry's growth
Heat-Stable Vaccine Formulations Market Analysis
- Heat-stable vaccine formulations, designed to remain effective without strict cold chain requirements, are becoming essential components in global immunization efforts across both human and veterinary healthcare settings due to their enhanced shelf life, distribution flexibility, and suitability for resource-limited environments
- The escalating demand for heat-stable vaccines is primarily fueled by increasing global vaccination programs, growing emphasis on pandemic preparedness, and the need to reduce cold chain logistics costs, especially in remote and low-income regions
- North America dominated the heat-stable vaccine formulations market with the largest revenue share of 37.6% in 2024, attributed to strong R&D investments, advanced pharmaceutical manufacturing infrastructure, and the presence of leading biotechnology companies focusing on vaccine innovation. The U.S. accounted for the majority share in the region due to early regulatory support, high immunization coverage, and accelerated development of thermostable vaccine technologies for COVID-19 and other infectious diseases
- Asia-Pacific is projected to be the fastest-growing region in the heat-stable vaccine Formulations market during the forecast period, with a CAGR of 19.2% from 2025 to 2032, driven by increasing urbanization, rising government healthcare expenditures, expanding vaccine production capabilities in countries such as India and China, and rising demand for cost-effective and logistically feasible immunization solutions
- The Live Attenuated Vaccine segment dominated the heat-stable vaccine formulations market with a market share of 43.2% in 2024, driven by its established efficacy and widespread use in preventing diseases such as measles, mumps, rubella, and rotavirus. These vaccines are increasingly being reformulated using thermostabilization techniques to overcome cold chain dependency, especially for mass immunization programs in developing nations
Report Scope and Heat-Stable Vaccine Formulations Market Segmentation
|
Attributes |
Heat-Stable Vaccine Formulations Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Heat-Stable Vaccine Formulations Market Trends
“Improved Accessibility and Distribution in Resource-Limited Settings”
- A significant and accelerating trend in the global heat-stable vaccine formulations market is the development of vaccines that maintain efficacy and safety even without strict cold chain requirements, which is enhancing accessibility in low-resource and remote regions
- For instance, heat-stable formulations of vaccines such as the rotavirus and COVID-19 vaccines are enabling healthcare providers to immunize populations in areas with limited refrigeration infrastructure. This advancement is crucial for increasing immunization coverage in developing countries
- These formulations reduce dependency on cold storage logistics, simplify transportation, and minimize vaccine wastage, especially in areas affected by frequent power outages or insufficient healthcare infrastructure
- The growing focus from organizations such as WHO, GAVI, and UNICEF on improving global immunization programs has amplified demand for heat-stable vaccines. Collaborative efforts and funding are fueling R&D for more thermostable vaccines targeting diseases such as measles, polio, and tuberculosis
- Companies are leveraging innovative technologies such as spray drying, lyophilization, and nanoemulsion-based delivery systems to enhance vaccine stability at ambient temperatures. These approaches enable mass immunization campaigns without heavy reliance on cold chain systems
- The demand for heat-stable vaccine formulations is rapidly growing across both humanitarian health missions and commercial pharmaceutical distribution, as global health organizations and governments prioritize universal vaccine access and outbreak preparedness
Heat-Stable Vaccine Formulations Market Dynamics
Driver
“Growing Need Due to Expanding Global Immunization and Cold Chain Limitations”
- The increasing focus on equitable global immunization, especially in remote and low-resource regions, coupled with the challenges of maintaining cold chain logistics, is a significant driver for the rising demand for heat-stable vaccine formulations
- For instance, in April 2024, the World Health Organization (WHO) partnered with leading pharmaceutical manufacturers to pilot thermostable measles-rubella vaccine distribution in sub-Saharan Africa, significantly reducing vaccine spoilage and improving outreach during mobile immunization drives. Such initiatives are expected to drive the Heat-Stable Vaccine Formulations industry growth in the forecast period
- As healthcare providers and global health organizations seek more reliable and accessible vaccination solutions, heat-stable vaccines offer advanced benefits such as longer shelf life, simplified transportation, and better resilience in harsh environmental conditions—making them a compelling upgrade over traditional cold-chain-dependent vaccines
- Furthermore, the growing emphasis on pandemic preparedness and emergency response readiness is pushing demand for vaccines that can be stockpiled and deployed rapidly without refrigeration. These attributes make heat-stable formulations integral to national immunization programs and disaster response strategies
- The convenience of storing and administering vaccines without cold chain dependency, especially in conflict zones, disaster-hit areas, and developing nations, is a key factor driving their adoption. The trend toward decentralized healthcare and field-based vaccination campaigns further contributes to market growth
Restraint/Challenge
“Challenges in Manufacturing Scalability and Regulatory Hurdles”
- Despite strong benefits, the scalability of manufacturing heat-stable vaccine formulations presents a significant challenge due to the technical complexity of maintaining vaccine efficacy under elevated temperatures. Reformulating vaccines without compromising potency or immunogenicity requires extensive R&D and robust stability testing
- For instance, delays in achieving WHO prequalification for certain thermostable rotavirus and hepatitis B vaccine candidates have slowed deployment in some target regions, highlighting regulatory and scientific barriers
- Addressing these challenges through investments in formulation science, partnerships with biotech startups, and collaboration with regulatory agencies is crucial to ensure reliable quality and market accessibility. In addition, manufacturing costs for specialized formulation technologies—such as spray drying, film-coating, or encapsulation—can be higher than traditional methods, presenting an adoption barrier for price-sensitive markets
- While global health organizations provide funding support, the perceived premium of heat-stable vaccines can deter adoption in regions already struggling with limited healthcare budgets or procurement constraints
- Overcoming these hurdles through process innovation, technology transfer to local manufacturers, and the introduction of hybrid cold-chain + heat-stable solutions will be essential for widespread implementation and long-term market growth
Heat-Stable Vaccine Formulations Market Scope
The heat-stable vaccine formulations market is segmented on the basis of type, formulation technology, stability mechanism, and application.
• By Type
On the basis of type, the heat-stable vaccine formulations market is segmented into live attenuated vaccines, inactivated vaccines, subunit vaccines, toxoid vaccines, mRNA vaccines, viral vector vaccines, and others. The live attenuated vaccines segment dominated with the largest revenue share of 43.2% in 2024, due to their high immunogenicity and single-dose efficacy, especially in global immunization campaigns.
The mRNA vaccines segment is expected to witness the fastest growth rate with a CAGR of 24.5% from 2025 to 2032, driven by continuous innovation in thermostable formulations and post-pandemic demand.
• By Formulation Technology
On the basis of formulation technology, the market is segmented into lyophilized (freeze-dried), spray-dried, film-based, encapsulation-based, and others. The lyophilized (freeze-dried) segment held the largest revenue share of 35.6% in 2024, attributed to its proven success in prolonging shelf life and minimizing cold chain dependence.
The spray-dried segment is projected to grow at the fastest rate, with a CAGR of 22.1% from 2025 to 2032, owing to its cost-effectiveness, ease of transport, and increasing adoption for pediatric and oral vaccine delivery.
• By Stability Mechanism
On the basis of stability mechanism, the market is segmented into thermostable by excipients, thermostable by drying method, thermostable by packaging, and others. The thermostable by excipients segment accounted for the largest share of 38.4% in 2024, with excipients such as trehalose, sucrose, and mannitol playing a pivotal role in preserving vaccine potency under heat exposure.
The thermostable by packaging segment is expected to register the highest growth, with a CAGR of 21.4% from 2025 to 2032, driven by innovations in multilayer vials, aluminum-based blister packs, and desiccant-lined packaging.
• By Application
On the basis of application, the heat-stable vaccine formulations market is segmented into human vaccination, veterinary vaccination, pandemic preparedness, routine immunization programs, and others. The human vaccination segment dominated the market with a revenue share of 49.2% in 2024, led by large-scale immunization initiatives in emerging economies and global efforts to reduce cold chain dependency.
The pandemic preparedness segment is expected to witness the fastest expansion with a CAGR of 25.6% from 2025 to 2032, fueled by global health security agendas, WHO support, and the demand for ready-to-deploy vaccines in emergency scenarios.
Heat-Stable Vaccine Formulations Market Regional Analysis
- North America dominated the heat-stable vaccine formulations market with the largest revenue share of 37.6% in 2024, driven by rising demand for cold chain-independent vaccines, robust healthcare infrastructure, and strong funding from government and private organizations for immunization programs
- The region benefits from advanced pharmaceutical research, high awareness of vaccine stability challenges, and early adoption of novel formulation technologies
- This widespread growth is further supported by favorable regulatory frameworks, a focus on pandemic preparedness, and the presence of leading biotech companies investing in thermostable vaccine development
U.S. Heat-Stable Vaccine Formulations Market Insight
The U.S. heat-stable vaccine formulations market captured the largest revenue share of 61% in 2024 within North America, driven by strong R&D investments, increasing demand for efficient vaccine delivery in remote regions, and accelerated deployment of vaccines during emergency health crises. The market is also supported by initiatives from federal agencies and organizations such as BARDA and NIH to reduce cold chain dependency, especially for stockpiling vaccines for national security and outbreak readiness.
Europe Heat-Stable Vaccine Formulations Market Insight
The Europe heat-stable vaccine formulations market is projected to expand at a substantial CAGR throughout the forecast period, fueled by the growing focus on sustainable healthcare logistics and the need to reduce vaccine wastage during transportation and storage. European Union health authorities are increasingly funding programs that support the development and distribution of thermostable vaccines, particularly in Eastern Europe and underserved rural areas. The region's strong biopharmaceutical sector, combined with public-private collaborations, continues to accelerate research into next-generation vaccine formulations.
U.K. Heat-Stable Vaccine Formulations Market Insight
The U.K. market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by the government's proactive immunization strategy and its emphasis on vaccine equity. The U.K. is also investing in global health initiatives that promote heat-stable vaccine deployment in low-income countries through partnerships with organizations such as Gavi and CEPI, thereby encouraging domestic innovation.
Germany Heat-Stable Vaccine Formulations Market Insight
The Germany heat-stable vaccine formulations market is expected to expand at a considerable CAGR during the forecast period, backed by the country’s strong emphasis on research, innovation, and sustainability in pharmaceutical manufacturing. Germany is emerging as a key hub for the development of thermostable mRNA and subunit vaccines, with both private companies and academic institutions actively engaged in formulation R&D.
Asia-Pacific Heat-Stable Vaccine Formulations Market Insight
The Asia-Pacific heat-stable vaccine formulations market is projected to grow at the fastest CAGR of 19.2% from 2025 to 2032, driven by rising population density, expanding immunization campaigns, and limited access to cold storage infrastructure in several emerging economies. Countries such as India, China, and Indonesia are heavily investing in heat-stable formulations to improve rural vaccination rates and reduce logistics costs, with support from global health agencies and vaccine alliances.
Japan Heat-Stable Vaccine Formulations Market Insight
The Japan heat-stable vaccine formulations market is gaining momentum due to the country’s focus on self-reliance in vaccine production and pandemic preparedness. With a technologically advanced pharmaceutical sector, Japan is exploring heat-stable formulations for emergency stockpiling and export to Asia-Pacific neighbors, contributing to the market’s steady growth.
China Heat-Stable Vaccine Formulations Market Insight
The China heat-stable vaccine formulations market held the largest revenue share in the Asia-Pacific region in 2024, driven by government incentives for local production of vaccines, rising export activities, and strategic partnerships between domestic players and global pharmaceutical firms. China is positioning itself as a key supplier of heat-stable vaccines, particularly for developing countries in Africa and Southeast Asia, benefiting from economies of scale and cost-effective manufacturing.
Heat-Stable Vaccine Formulations Market Share
The heat-stable vaccine formulations industry is primarily led by well-established companies, including:
- Serum Institute of India Pvt. Ltd. (India)
- Bharat Biotech (India)
- GSK plc (U.K.)
- Sanofi (France)
- Pfizer Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Johnson & Johnson and its affiliates (U.S.)
- Novavax, Inc. (U.S.)
- Moderna, Inc. (U.S.)
- AstraZeneca plc (U.K.)
- CureVac N.V. (Germany)
- Emergent BioSolutions Inc. (U.S.)
- Biological E Limited (India)
- Innovative Biotech Ltd. (Nigeria)
- Valneva SE (France)
- CSL Limited (Australia)
- IDT Biologika GmbH (Germany)
- Chongqing Zhifei Biological Products Co., Ltd. (China)
- China National Pharmaceutical Group Corporation (Sinopharm) (China)
- Zydus Lifesciences Ltd. (India)
Latest Developments in Global Heat-Stable Vaccine Formulations Market
- In March 2025, Bavarian Nordic (Denmark/USA) received FDA approval for a freeze-dried formulation of its Jynneos vaccine (for mpox and smallpox), enabling improved stability and logistics for stockpiling and emergency deployment
- In April 2025, Stablepharma Ltd (UK) commenced a Phase I clinical trial of SPVX02, the world’s first “fridge‑free” tetanus‑diphtheria booster, at the NIHR Southampton Clinical Research Facility—marking a pioneering effort in human trials
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.