Global Her2 Gastric Cancer Market
Market Size in USD Billion
CAGR :
% 
 USD
1.32 Billion
USD
1.86 Billion
2024
2032
USD
1.32 Billion
USD
1.86 Billion
2024
2032
| 2025 –2032 | |
| USD 1.32 Billion | |
| USD 1.86 Billion | |
|
|
|
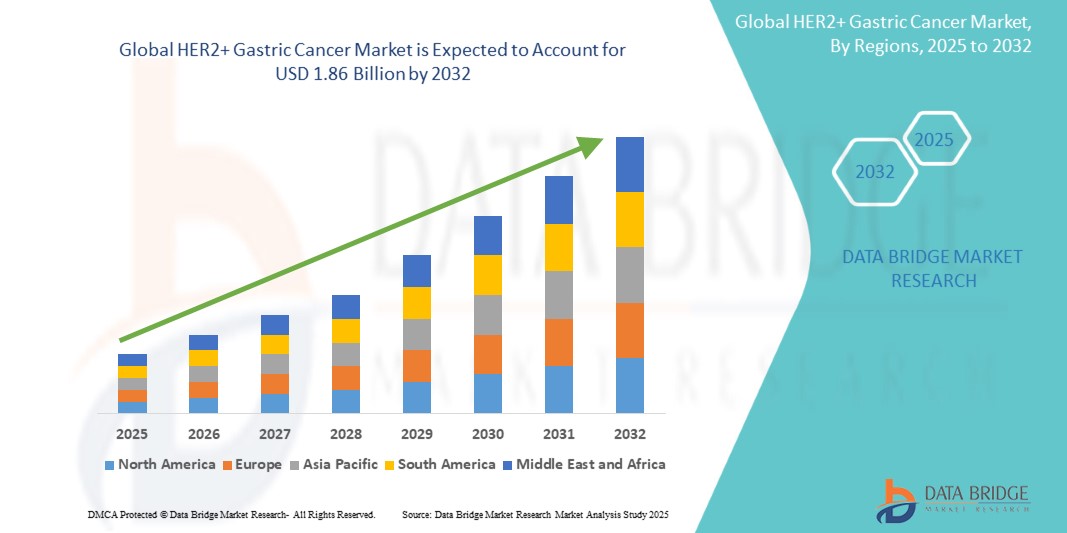
HER2+ Gastric Cancer Market Size
- The global HER2+ Gastric Cancer market was valued at USD 1.32 billion in 2024 and is expected to reach USD 1.86 billion by 2032
- During the forecast period of 2025 to 2032 the market is sly to grow at a CAGR of 4.44%, primarily driven by the rising prevalence and targeted therapies
- This growth is driven by factors such as the advancement in targeted therapies and rising prevalence and early diagnosis
HER2+ Gastric Cancer Market Analysis
- HER2+ gastric cancer refers to a subtype of gastric cancer characterized by an overexpression of the HER2 (human epidermal growth factor receptor 2) protein. This protein promotes cancer cell growth, and its overexpression can lead to more aggressive tumor behavior. HER2+ gastric cancer is often diagnosed through biopsy or blood tests that detect the presence of high HER2 levels on cancer cells
- The HER2+ gastric cancer market’s growth is primarily driven by the increasing prevalence of the condition and advancements in targeted therapies. Targeted therapies, such as trastuzumab (Herceptin), have significantly improved patient outcomes by specifically targeting HER2 receptors on cancer cells, preventing tumor growth and spread. As more treatments are developed to target HER2, and as early diagnosis improves, the demand for these therapies is expected to rise
- For instance, In June 2021, FDA approved trastuzumab deruxtecan (Enhertu) for the treatment of HER2-positive gastric cancer. This approval was based on clinical trial data showing significant improvements in progression-free survival and overall response rates, offering a new targeted treatment option for patients with advanced HER2+ gastric cancer
- Moreover, an aging population and lifestyle factors contributing to gastric cancer incidence are expected to further drive market growth. Ongoing research and the development of novel HER2-targeted therapies will likely continue to shape the landscape of HER2+ gastric cancer treatment
Report Scope and HER2+ Gastric Cancer Market Segmentation
|
Attributes |
HER2+ Gastric Cancer Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
HER2+ Gastric Cancer Market Trends
“Increasing Focus on Combination Therapies”
- One notable trend in the HER2+ gastric cancer market is the increasing focus on combination therapies. Researchers and healthcare providers are exploring the synergy between HER2-targeted therapies and other treatment modalities, such as chemotherapy, immune checkpoint inhibitors, and other novel biologics. This trend aims to improve patient outcomes by addressing tumor heterogeneity and overcoming resistance mechanisms that may limit the effectiveness of single-agent therapies
- For instance, In August 2020, HER2-targeted therapy trastuzumab deruxtecan (Enhertu), was approved for patients with HER2-positive metastatic gastric cancer. Clinical trials have shown that when combined with chemotherapy or other drugs, it significantly enhances progression-free survival
- The positive results from these studies have spurred more research into multi-drug combinations to maximize therapeutic efficacy and improve overall survival rates in patients with advanced HER2+ gastric cancer. This approach is expected to drive market growth in the coming years
HER2+ Gastric Cancer Market Dynamics
Driver
“Advancement in Targeted Therapies”
- One significant driver of the HER2+ gastric cancer market is the advancement in targeted therapies, which are revolutionizing the treatment landscape
- Targeted therapies specifically focus on the HER2 protein, which is overexpressed in a subset of gastric cancers, offering more precise and effective treatment compared to traditional chemotherapy. These therapies aim to block the growth and spread of cancer cells by targeting HER2 receptors, thus improving patient outcomes and reducing side effects
For Instance,
- In June 2021, the FDA approved trastuzumab deruxtecan (Enhertu) for HER2-positive gastric cancer patients. This approval was based on a clinical trial showing that trastuzumab deruxtecan significantly improved progression-free survival and response rates compared to chemotherapy. This approval marked a major milestone in the treatment of advanced HER2+ gastric cancer, providing a more targeted and effective treatment option for patients with limited choices
- In December 2022, the European Medicines Agency (EMA) approved HER2-targeted therapy zanidatamab for advanced gastric cancer. This approval was based on positive clinical trial results, showing significant improvements in overall survival and response rates when zanidatamab was combined with other therapies. The approval further solidified the trend of targeting HER2+ tumors with highly specific therapies, offering hope to patients with advanced stages of gastric cancer
- These approvals highlight the growing momentum in the development of HER2-targeted therapies, creating a robust pipeline and driving market growth. With continuous advancements in research, as well as an increasing number of novel treatments targeting HER2, the HER2+ gastric cancer market is expected to expand rapidly, ultimately improving patient survival rates and reducing the global burden of this aggressive cancer
- The market growth is directly linked to these innovations, providing better treatment options and more favorable outcomes for patients
Opportunity
“Expansion of Treatment Options Through Novel Combination Therapies”
- One significant opportunity in the HER2+ gastric cancer market is the expansion of treatment options through novel combination therapies. As research continues to reveal the potential synergy between HER2-targeted therapies and other treatment modalities, such as immunotherapy and chemotherapy, there is a growing opportunity to enhance patient outcomes
- Combining these treatments can improve efficacy, minimize resistance, and address cancer’s ability to evolve and escape conventional treatments. This approach is particularly beneficial for patients with advanced or refractory HER2+ gastric cancer, for whom treatment options are limited
For Instance,
- In September 2020, the combination of trastuzumab (Herceptin), a HER2-targeted monoclonal antibody, and chemotherapy was shown to significantly improve overall survival rates in patients with HER2+ gastric cancer in clinical trials. The combination therapy was approved by the FDA, offering patients a more effective treatment regimen compared to single-agent chemotherapy. This combination has become a standard of care in treating HER2+ gastric cancer
- In January 2022, a study presented at the American Association for Cancer Research (AACR) Annual Meeting demonstrated the promising effects of combining immune checkpoint inhibitors, such as pembrolizumab (Keytruda), with HER2-targeted therapies. The study showed that this combination could enhance immune system activity and further inhibit tumor growth. This approach is still under investigation but offers significant potential for improving treatment outcomes in patients with advanced stages of the disease
- These instances highlight the potential for combination therapies to address treatment resistance and improve the overall effectiveness of HER2+ gastric cancer treatments. As research continues to uncover more effective combinations, the HER2+ gastric cancer market is expected to grow substantially. With more treatment options becoming available, the market is poised for expansion, offering hope for better clinical outcomes and driving sustained growth in the coming years
Restraint/Challenge
“High Treatment Cost limiting Patient Access to Novel Therapies”
- One key restraint in the HER2+ gastric cancer market is the high treatment cost, which can limit patient access to novel therapies and slow down market growth. Despite the effectiveness of targeted treatments such as trastuzumab deruxtecan (Enhertu) and other HER2-specific therapies, their expensive price tags pose a significant barrier, especially in low- and middle-income countries
- These high costs can lead to delayed treatment initiation, reduced patient adherence, and, in some cases, patients being unable to access the most effective therapies
For Instance,
- In August 2020, the approval of trastuzumab deruxtecan (Enhertu) by the FDA for HER2-positive gastric cancer provided a new treatment option. However, the drug's price remained a concern, with costs exceeding USD 100,000 per year. This has raised challenges for both healthcare providers and patients, especially in countries without adequate insurance coverage or reimbursement schemes, limiting its widespread adoption
- In May 2021, Zanidatamab, another HER2-targeted therapy, was under review by regulatory agencies for HER2+ gastric cancer. Despite its promising results in clinical trials, the therapy's high cost was flagged as a major limitation to its accessibility, particularly in regions where healthcare systems are under pressure or where insurance coverage is limited
- These instances highlight the ongoing challenge of treatment affordability for HER2+ gastric cancer therapies. The high costs can limit market penetration, especially in underserved regions, slowing down the broader adoption of these innovative treatments
- While the efficacy of these drugs remains a strong market driver, the financial burden associated with them acts as a restraint, potentially hindering the overall growth of the HER2+ gastric cancer market. As such, more cost-effective treatment options or reimbursement solutions will be crucial for sustaining market expansion in the future
HER2+ Gastric Cancer Market Scope
The market is segmented on the basis of stage, therapy, and end-user.
|
Segmentation |
Sub-Segmentation |
|
By Stage |
|
|
By Therapy |
|
|
By End-User |
|
HER2+ Gastric Cancer Market Regional Analysis
“North America is the Dominant Region in the HER2+ Gastric Cancer Market”
- North America dominates the HER2+ Gastric Cancer Market, driven by the advanced healthcare systems, high adoption of innovative treatments, and strong reimbursement policies that facilitate access to targeted therapies
- In addition, the presence of leading pharmaceutical companies and ongoing clinical research contribute to the growth of the market in this region. The increasing prevalence of HER2+ gastric cancer and improved survival rates further drive the market in North America
- U.S. in particular dominates this market which is due to the country’s advanced healthcare infrastructure, high adoption rates of targeted therapies, and strong pharmaceutical research and development capabilities. In addition, the U.S. has access to cutting-edge treatments such as trastuzumab (Herceptin) and trastuzumab deruxtecan, which are crucial for HER2+ gastric cancer management
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is projected to register the highest growth rate in the HER2+ gastric cancer market. Countries such as China, Japan, and South Korea are witnessing rising incidence rates of gastric cancer, particularly HER2-positive cases, due to lifestyle factors and aging populations
- In addition, there is increasing awareness and accessibility to advanced treatments such as HER2-targeted therapies. The region's improving healthcare infrastructure, growing medical research investments, and expanding access to innovative therapies are expected to drive significant growth in the HER2+ gastric cancer market, making it the fastest-growing region in the coming years
- China is projected to register the highest growth rate in the HER2+ gastric cancer market. The country has a high incidence of gastric cancer, especially in the HER2-positive subtype, and an aging population, which is contributing to the rising demand for targeted therapies
HER2+ Gastric Cancer Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- AstraZeneca (U.K.)
- ALX Oncology Inc. (U.S.)
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Ambrx (U.S.)
- Amgen Inc. (U.S.)
- Astellas Pharma Inc. (Japan)
- Bayer AG (Germany)
- BeiGene LTD (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- Genentech, Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Lilly (U.S.)
- Merck & Co., Inc. (U.S.)
- MacroGenics, Inc. (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- TAIHO PHARMACEUTICAL CO., LTD. (Japan)
- Zai Lab (China)
Latest Developments in Global HER2+ Gastric Cancer Market
- In March 2025, the Food and Drug Administration (FDA) granted traditional approval for pembrolizumab (Keytruda, Merck) in combination with trastuzumab, fluoropyrimidine-, and platinum-based chemotherapy for the first-line treatment of adults with locally advanced, unresectable, or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors express PD-L1 (CPS ≥1)
- In January 2025, ALX Oncology Holdings Inc. announced positive updated results from the ASPEN-06 Phase 2 clinical trial, showing that the company’s investigational CD47-blocker, evorpacept, produces a durable clinical response and has a well-tolerated safety profile in patients with previously treated HER2-positive advanced gastric cancer (GC) or gastroesophageal junction (GEJ) cancer
- In January 2025, Astellas Pharma Inc. announced that China’s National Medical Products Administration (NMPA) had approved VYLOY (zolbetuximab), in combination with fluoropyrimidine- and platinum-based chemotherapy, for the first-line treatment of patients with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors are positive for claudin (CLDN) 18.2
- In December 2024, BeiGene, Ltd. announced that the U.S. Food and Drug Administration (FDA) had approved TEVIMBRA (tislelizumab-jsgr), in combination with platinum and fluoropyrimidine-based chemotherapy, for the first-line treatment of unresectable or metastatic HER2-negative gastric or gastroesophageal junction (G/GEJ) adenocarcinoma in dults whose tumors express PD-L1 (≥1)
- In August 2024, AstraZeneca and Daiichi Sankyo’s Enhertu (trastuzumab deruxtecan) received conditional approval in China as a monotherapy for treating adult patients with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma who have undergone two or more prior treatment regimens. This conditional approval from the National Medical Products Administration (NMPA) was based on the positive outcomes of the DESTINY-Gastric06 Phase II trial
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.