Global Herceptin Biosimilars Market
Market Size in USD Million
CAGR :
% 
 USD
1,810.00 Million
USD
11,302.00 Million
2022
2030
USD
1,810.00 Million
USD
11,302.00 Million
2022
2030
| 2023 –2030 | |
| USD 1,810.00 Million | |
| USD 11,302.00 Million | |
|
|
|
|
Herceptin Biosimilars Market Analysis and Size
The incidence of Chronic Lymphocytic Leukemia (CLL) is around 21,000 new cases per year in the U.S. In comparison, the estimated incidence of non-Hodgkin lymphoma (NHL) is around 77,000 new cases per year in the U.S., the incidence of acute lymphoblastic leukemia (ALL) is much lower, with an estimated 6,000 new cases per year in the United States.
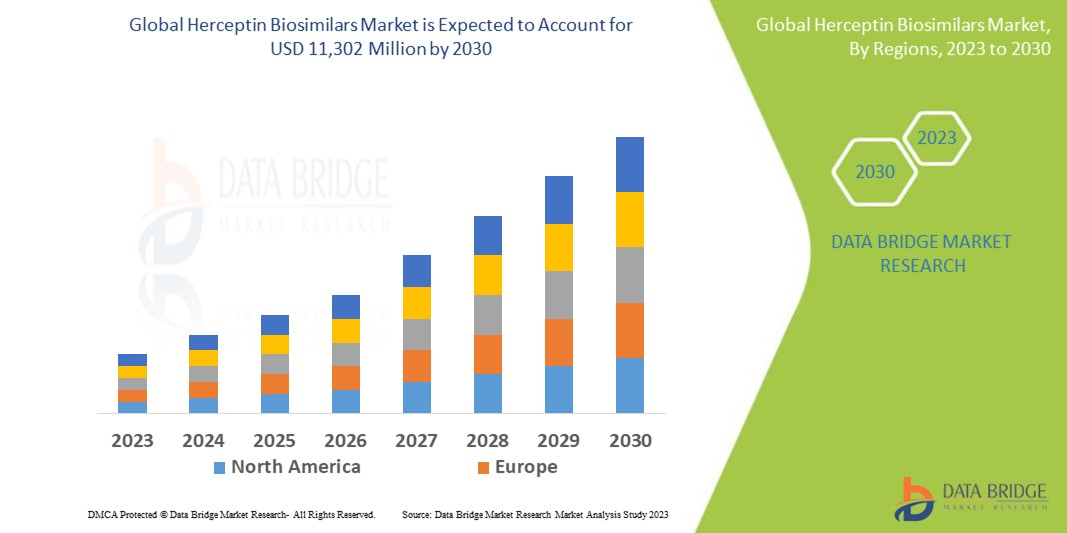
Data Bridge Market Research analyses that the market which was USD 1,810 million in 2022, would rocket up to USD 11,302 million by 2030 and is expected to undergo a CAGR of 23.2% during the forecast period. This indicates the market value. “Breast Cancer” dominates the application segment of the Herceptin Biosimilars market owing to the growing prevalence of breast cancer globally. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Herceptin Biosimilars Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, and Pricing in USD |
|
Segments Covered |
By Application (Breast Cancer, Colorectal Cancer, Leukemia, Lymphoma, and Others), Dosage (150mg/single-dose vial and 420mg/multidose vial), End-Users (Specialty Clinics, Hospitals, Oncology Centers, and Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, and Others) |
|
Countries Covered |
U.S., Canada, Mexico, Brazil, Argentina, Peru, rest of South America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Vietnam, rest of Asia-Pacific, Saudi Arabia, U.A.E., Egypt, Israel, Kuwait, South Africa, rest of Middle East and Africa. |
|
Market Players Covered |
Amgen Inc. (U.S), AryoGen Pharmed (Iran), Biocon (India), Accord Healthcare Ltd (U.K), Celltrion Healthcare Co.,Ltd. (South Korea), Pfizer Inc. (U.S), Merck Sharp & Dohme Corp., A subsidiary of Merck & Co., Inc. (U.S), Shanghai Henlius Biotech, Inc. (China), Gedeon Richter Plc. (Hungary), Genor Biopharma Company Ltd (China), MABION S.A (Poland), Mylan N.V. (U.K), F. Hoffmann-La Roche Ltd (Switzerland), Samsung Bioepis (South Korea), ACROBiosystems (U.S), Genentech, USA Inc. (U.S), AstraZeneca (U.K), Halozyme, Inc. (U.S), and Teva Pharmaceuticals USA, Inc. (U.S) among others |
|
Market Opportunities |
|
Market Definition
Herceptin (Trastuzumab) is a monoclonal antibody that provides effective results by binding with the human epidermal growth factor receptor 2 (HER2). In several types of cancer, the HER2 gene is overexpressed, leading to cancer development due to the uncontrollable growth of cells. Herceptin received its first approval in 1998, indicated for treating metastatic breast cancer. Now approximately five Herceptin biosimilar drugs have been approved in the U.S. market. Herceptin biosimilar drug is available for USD 70,000 for one year of treatment and has been proven as the standard treatment of breast cancer.
Global Herceptin Biosimilars Market Dynamics
Drivers
- Rising Incidence of Breast Cancer
The market for Herceptin Biosimilars is anticipated to grow significantly over the forecast period due to a number of key factors, including the rising prevalence of cancer diseases specifically breast cancer. Herceptin biosimilars can help meet the growing demand for treatment of HER-2 Positive breast cancer.
- Increasing Access to Therapy and Cost Effectiveness
The availability of Herceptin biosimilars can increase access to therapy for patients with HER2-positive breast cancer, as it can reduce the financial burden of treatment. Also, Herceptin biosimilars are generally priced lower than the originator drug, which can lead to significant cost savings for healthcare systems, patients, and payers. These factors are responsible for driving the market in the forecast period.
Opportunities
- Emerging Markets Creating Opportunities
Emerging markets, such as Asia-Pacific and Latin America, offer significant growth opportunities for the Herceptin biosimilars market due to the growing incidence of breast cancer and increasing demand for cost-effective treatments.
- Patient Expirations
The patent expiration of other biologics in the HER2-positive breast cancer market, such as Perjeta and Kadcyla, can create opportunities for Herceptin biosimilars to gain substantial market shares.
Restraints/Challenges
- Limited Awareness
The limited awareness regarding Herceptin use and stringent regulations for approval and manufacturing of Herceptin biosimilars are the factors that will hinder the market growth and will further challenge the Herceptin biosimilars. Also, lack of favorable reimbursement scenario and technology penetration in the developing economies, safety concerns with issues of Herceptin biosimilar and lack of suitable infrastructure in low- and middle-income countries are projected to challenge the market in the forecast period of 2023-2030.
This Herceptin Biosimilars market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the Herceptin Biosimilars market contact Data Bridge Market Research for an analyst brief, our team will help you make an informed market decision to achieve market growth.
Recent Developments
- In July 2021, Zercepac (trastuzumab-dkst) by Teva Pharmaceuticals was approved by the US FDA for the treatment of HER2-positive breast cancer and HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma
- In January 2021, Samsung Bioepis announced that Ontruzant (SB3) had been approved by the European Commission for the treatment of HER2-positive breast cancer
- In January 2020, Celltrion Healthcare announced that the European Commission approved their drug Herzuma (CT-96) for the treatment of HER2-positive breast cancer
- In June 2019, Kanjinti (trastuzumab-anns) by Amgen and Allergan was approved by the US FDA for the treatment of HER2-positive breast cancer and HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma.
Global Herceptin Biosimilars Market Scope
The Herceptin Biosimilars market is segmented on the basis of application, dosage, end-users and distribution channel. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core applications.
Application
- Breast Cancer
- Colorectal Cancer
- Leukemia
- Lymphoma
- Others
Dosage
- 150mg/single-dose vial
- 420mg/multidose vial
End Users
- Specialty Clinics
- Hospitals
- Oncology Centers
- Others
Distribution Channel
- Direct Tender
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Global Herceptin Biosimilars Market Regional Analysis/Insights
The market is analysed and market size insights and trends are provided by country, application, dosage, end-users, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada, Mexico, Brazil, Argentina, Peru, rest of South America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Vietnam, rest of Asia-Pacific, Saudi Arabia, U.A.E., Egypt, Israel, Kuwait, South Africa, rest of Middle East and Africa.
North America dominates the market because of its strong base of healthcare facilities, the strong presence of major players, the extraordinary healthcare infrastructure, and the large pool of people having breast cancer, Leukemia, Lymphoma, and others.
Asia-Pacific is expected to witness significant growth during the forecast period of 2023 to 2030 due to the increase in government initiatives to promote healthcare, the rising health awareness among the people and growing demand for advanced medical technology for cancer treatment, the large population pool, and the growing demand for quality healthcare in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends, and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of domestic tariffs, and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The Herceptin Biosimilars market also provides you with a detailed market analysis for every country's growth in healthcare expenditure for capital equipment, installed base of different kinds of products for the Herceptin Biosimilars market, the impact of technology using lifeline curves and changes in healthcare regulatory scenarios and their impact on the Herceptin Biosimilars market.
Competitive Landscape and Herceptin Biosimilars Market Share Analysis
The Herceptin Biosimilars market competitive landscape provides details by competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width, and breadth, application dominance. The above data points provided are only related to the companies' focus related to the Herceptin Biosimilars market.
Some of the major players operating in the market are:
- Amgen Inc. (U.S.)
- AryoGen Pharmed (Iran)
- Accord Healthcare Ltd (U.K.)
- Biocon (India)
- Celltrion Healthcare Co. Ltd. (South Korea)
- Pfizer Inc. (U.S.)
- Merck Sharp & Dohme Corp., A subsidiary of Merck & Co., Inc. (U.S.)
- Shanghai Henlius Biotech, Inc. (China)
- Gedeon Richter Plc. (Hungary)
- Genor Biopharma Company Ltd (China)
- MABION S.A (Poland)
- Mylan N.V. (U.K.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Samsung Bioepis (South Korea)
- ACROBiosystems (U.S.)
- Genentech, USA Inc. (U.S.)
- AstraZeneca (U.K.)
- Halozyme, Inc. (U.S.)
- Teva Pharmaceuticals USA, Inc. (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.