Global Hidradenitis Suppurativa Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
799.90 Billion
USD
1,154.47 Billion
2024
2032
USD
799.90 Billion
USD
1,154.47 Billion
2024
2032
| 2025 –2032 | |
| USD 799.90 Billion | |
| USD 1,154.47 Billion | |
|
|
|
|
Hidradenitis Suppurativa Treatment Market Size
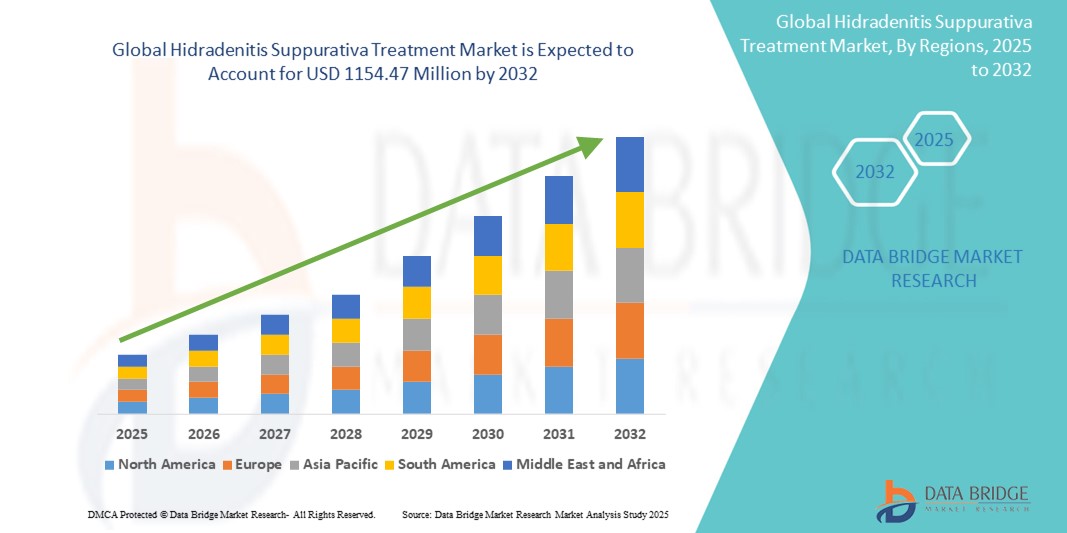
- The global hidradenitis suppurativa treatment market size was valued at USD 799.49 million in 2024 and is expected to reach USD 1154.47 million by 2032, at a CAGR of 4.70% during the forecast period
- This growth is driven by increasing awareness about chronic skin diseases, rising demand for biologics, and improved access to dermatological care
Hidradenitis Suppurativa Treatment Market Analysis
- Hidradenitis Suppurativa (HS) is a painful, long-term skin condition characterized by inflamed nodules and abscesses
- The market for HS treatments is witnessing strong growth due to the rising diagnosis rate, demand for targeted therapies, and increasing clinical trials evaluating biologics
- North America dominates the hidradenitis suppurativa treatment market with a market share of approximately 43.2%, supported by advanced dermatology infrastructure, high treatment awareness, and strong reimbursement frameworks
- Asia-Pacific is projected to grow at the fastest pace and currently holds an estimated market share of 21.6%, driven by increasing dermatology consultations, improved public health spending, and rising awareness of chronic skin disorders
- The medication segment is expected to capture a market share of 61.3%, driven by the increasing adoption of pharmacological treatments for both acute flare management and long-term control of hidradenitis suppurativa
Report Scope and Hidradenitis Suppurativa Treatment Market Segmentation
|
Attributes |
Hidradenitis Suppurativa Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Hidradenitis Suppurativa Treatment Market Trends
" Increased Adoption of Biologics in Moderate to Severe HS Cases”
- Biologic therapies, particularly adalimumab (Humira), the only FDA-approved biologic for hidradenitis suppurativa (HS), are now standard care for moderate to severe cases, targeting TNF-α to reduce inflammation and lesion formation
- Clinical trials are exploring novel agents like secukinumab (IL-17 inhibitor) and bimekizumab (IL-17A/F inhibitor), with Phase III data showing up to 50% reduction in abscess and nodule counts. These advancements are expanding treatment options beyond traditional antibiotics (e.g., clindamycin) and surgery, aligning with the market’s shift toward precision medicine for chronic inflammatory conditions
- The integration of biologics into HS treatment guidelines, supported by EHSF (European Hidradenitis Suppurativa Foundation), is improving patient outcomes and reducing reliance on invasive procedures
- Ongoing research into IL-23 inhibitors, such as guselkumab, is further diversifying the biologic arsenal, offering hope for refractory cases
- For Instance, In 2024, Novartis’ Phase III SUNSHINE trial for secukinumab demonstrated a 45% HiSCR (Hidradenitis Suppurativa Clinical Response) rate at 16 weeks in moderate to severe HS patients, prompting EMA approval discussions in early 2025
- The growing use of biologics like adalimumab and emerging IL-17/IL-23 inhibitors is transforming HS treatment, improving outcomes for severe cases and fueling market expansion through targeted therapies
Hidradenitis Suppurativa Treatment Market Dynamics
Driver
"Rising Diagnosis Rate and Inclusion of HS in Dermatology Guidelines"
- Increased awareness through patient advocacy groups and dermatology campaigns has boosted HS diagnosis rates, with prevalence estimated at 1–4% globally. Standardization of diagnostic criteria, such as the Hurley staging system and IHS4 scoring, in guidelines by the American Academy of Dermatology (AAD) and EHSF has facilitated earlier detection in primary and specialty care
- This has led to higher treatment uptake, particularly for biologics and combination therapies, driving market growth in developed regions
- Public health campaigns, like the HS Foundation’s 2023 awareness month, have reduced stigma, encouraging patients to seek care. Improved diagnostic tools, including ultrasound for lesion assessment, are enhancing accuracy, further supporting treatment demand
- For Instance, A 2024 AAD initiative trained 2,000 U.S. dermatologists on HS diagnostic protocols, resulting in a 15% increase in reported HS cases by mid-2025
- Enhanced awareness and standardized diagnostics are significantly increasing HS diagnosis rates, driving treatment uptake and fostering market growth through early and accurate care
Opportunity
"Pipeline Expansion with Novel Immunomodulators"
- The HS treatment market is witnessing robust pipeline growth, with companies like Novartis, UCB, and Janssen developing novel immunomodulators, including IL-17 (secukinumab, bimekizumab) and IL-23 (guselkumab) inhibitors, to control chronic inflammation
- These therapies aim to achieve long-term symptom remission, addressing unmet needs in moderate to severe HS where antibiotics and surgery fall short
- Regulatory incentives, such as FDA Breakthrough Therapy Designations, are accelerating clinical development, expanding market opportunities
- Patient registries, like the Global HS Registry, are providing real-world data to guide trial design and therapy personalization
- These advancements are attracting investment and fostering collaborations, enhancing treatment accessibility in high-income markets
- For Instance, In February 2025, UCB’s bimekizumab received FDA Fast Track Designation for HS after Phase III data showed a 52% HiSCR rate at 24 weeks, with a planned 2026 launch
- The development of IL-17/IL-23 inhibitors and other immunomodulators is expanding the HS treatment pipeline, offering targeted solutions and driving market growth through innovative therapies
Restraint/Challenge
"Underdiagnosis and Delayed Referral in Primary Care Settings"
- HS is frequently underdiagnosed due to its symptom overlap with conditions like acne, folliculitis, or abscesses, leading to delays in referral to dermatologists
- Limited awareness among primary care providers and patients, coupled with stigma around painful, scarring lesions, results in an average diagnostic delay of 7–10 years, increasing disease severity and treatment costs
- This challenge restricts market growth, particularly in regions with fragmented healthcare systems
- In LMICs, lack of dermatology expertise and diagnostic tools like ultrasound exacerbates underdiagnosis. Efforts like EHSF’s 2024 provider training modules aim to improve recognition, but adoption remains uneven
- For Instance: A 2023 Journal of the American Academy of Dermatology study found that 65% of HS patients in rural U.S. areas were initially misdiagnosed, delaying biologic therapy by over 5 years
- Underdiagnosis and delayed referrals due to low awareness and symptom overlap pose significant barriers to the HS treatment market, necessitating improved education and diagnostic protocols
Hidradenitis Suppurativa Treatment Market Scope
The market is segmented on the basis of clinical stages, treatment type, route of administration, end users, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Clinical Stages |
|
|
By Treatment Type |
|
|
By Route of Administration |
|
|
By End User |
|
|
By Distribution Channel
|
|
In 2025, the medication is projected to dominate the market with a largest share in treatment type segment
In 2025, the medication segment is expected to capture a market share of 61.3%, driven by the increasing adoption of pharmacological treatments for both acute flare management and long-term control of Hidradenitis Suppurativa. Among these, anti-inflammatory drugs, antibiotics, corticosteroids, and especially biologics such as adalimumab (Humira®) have emerged as mainstays in clinical practice. The FDA approval of adalimumab for HS has positioned it as the gold standard, supported by robust clinical evidence and widespread physician endorsement. Additionally, newer biologics under investigation—including JAK inhibitors and IL-17 blockers—are expected to further boost the segment's growth.
The hospital segment expected to account for the largest share during the forecast period in end user segment
In 2025, the hospital segment is projected to command a market share of 57.6%, due to the central role hospitals play in the diagnosis, treatment, and long-term care of moderate-to-severe HS cases. Patients with chronic or advanced-stage HS are often referred early to hospitals for specialized care, including dermatology consultations, imaging, incision and drainage procedures, and biologic infusions. Hospitals also house multidisciplinary care teams—comprising dermatologists, wound care specialists, pain management experts, and surgeons—enabling comprehensive care for complex presentations.
Hidradenitis Suppurativa Treatment Market Regional Analysis
“North America Holds the Largest Share in the Hidradenitis Suppurativa Treatment Market”
- North America dominates the Hidradenitis Suppurativa Treatment market with a market share of approximately 43.2%, supported by advanced dermatology infrastructure, high treatment awareness, and strong reimbursement frameworks
- The U.S. leads the region with an estimated 34.1% share, driven by early diagnosis trends, favorable insurance coverage for biologics like adalimumab, and active participation in global clinical trials
- North America benefits from robust academic research institutions, established referral networks for chronic inflammatory skin diseases, and integrated dermatology-rheumatology clinics that manage severe HS cases
- The presence of biologic infusion centers, wound care programs, and surgical expertise within U.S. hospitals allows for comprehensive, multidisciplinary HS management
- The FDA’s support for expedited approvals of HS therapies has encouraged pipeline expansion and market availability of advanced biologics and small molecules.
- Major pharmaceutical players including AbbVie, Pfizer, Johnson & Johnson, and Bristol-Myers Squibb maintain significant R&D operations in the region, enabling rapid innovation and commercial rollout of new HS treatments.
“Asia-Pacific is Projected to Register the Highest CAGR in the Hidradenitis Suppurativa Treatment Market”
- Asia-Pacific is projected to grow at the fastest pace and currently holds an estimated market share of 21.6%, driven by increasing dermatology consultations, improved public health spending, and rising awareness of chronic skin disorders
- India and China are leading regional growth, supported by expansion in dermatology-focused hospitals, teledermatology platforms, and rising prescriptions of anti-inflammatory and biologic therapies
- Governments across the region are integrating skin health into public healthcare campaigns, enabling earlier detection and referral of HS cases, especially in urban centers
- Collaborative initiatives between public hospitals, dermatology associations, and private pharmaceutical firms are improving access to novel therapies and wound care products
- Countries such as Japan and South Korea are investing heavily in biologic innovation, clinical research, and specialist training, positioning themselves as regional leaders in HS treatment adoption
- Digital health adoption, medical tourism, and improved dermatological diagnostics are accelerating HS patient identification and long-term care in Asia-Pacific
Hidradenitis Suppurativa Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- AbbVie Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Novartis AG (Switzerland)
- GlaxoSmithKline plc (U.K.)
- Merck & Co., Inc. (U.S.)
- Sanofi S.A. (France)
- Bristol-Myers Squibb Company (U.S.)
- UCB S.A. (Belgium)
- Eli Lilly and Company (U.S.)
- LEO Pharma A/S (Denmark)
- Sun Pharmaceutical Industries Ltd. (India)
- Biocon Limited (India)
- AstraZeneca plc (U.K.)
- Incyte Corporation (U.S.)
- Aclaris Therapeutics, Inc. (U.S.)
- MoonLake Immunotherapeutics AG (Switzerland)
- Insmed Incorporated (U.S.)
- ACELYRIN, Inc. (U.S.)
- Avalo Therapeutics, Inc. (U.S.)
Latest Developments in Global Hidradenitis Suppurativa Treatment Market
- In January 2025, Roche announced positive Phase III trial results for a new bispecific antibody targeting cervical tumor microenvironment, showing a 27% improvement in progression-free survival over standard care
- In October 2024, GSK received EMA approval for its novel HPV-targeted immunotherapy for second-line cervical cancer treatment, expanding its oncology pipeline in Europe
- In August 2024, AstraZeneca launched a global clinical trial program for its next-generation PARP inhibitor in cervical cancer, involving over 20 countries and 3,000 patients
- In March 2024, Pfizer expanded its cervical cancer collaboration with academic institutions in India for biomarker-based treatment approaches and real-world data collection
- In November 2023, Merck & Co. partnered with a leading diagnostics firm to co-develop companion diagnostics for stratifying cervical cancer patients based on PD-L1 expression
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.