Global Hiv Diagnosis Market
Market Size in USD Billion
CAGR :
% 
 USD
2.86 Billion
USD
6.13 Billion
2022
2030
USD
2.86 Billion
USD
6.13 Billion
2022
2030
| 2023 –2030 | |
| USD 2.86 Billion | |
| USD 6.13 Billion | |
|
|
|
|
Human Immunodeficiency Virus (HIV) Diagnosis Market Analysis and Size
One of the major factors driving the increase in demand for Human immunodeficiency virus (HIV) systems is the rise in the prevalence of HIV infection. Rising expenditure for research and development proficiencies by the major healthcare companies coupled with increasing application of HIV antigen, nucleic acid testing and viral load testing will further generate lucrative and remunerative growth opportunities for the market. Rising adoption of advanced healthcare technological solutions and increased government initiatives to raise awareness will be important market growth determinants.
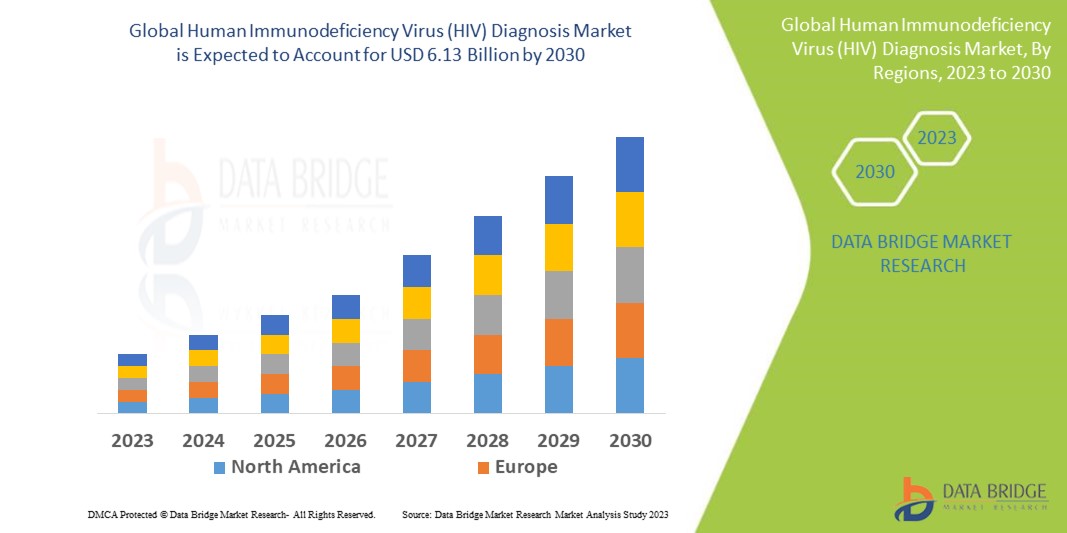
Data Bridge Market Research analyses that the human immunodeficiency virus (HIV) diagnosis market which is USD 2.86 billion in 2022, is expected to reach USD 6.13 billion by 2030, at a CAGR of 10.00% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Human Immunodeficiency Virus (HIV) Diagnosis Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Consumables, Assay, Kits and Reagents, Other Consumables, Instruments, Software and Services), Test Type (Antibody Tests, CD4 Count, Viral Load, Early Infant and Viral Identification), Application (Diagnostic Laboratories, Hospitals, Blood Banks, Home Care Settings and Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Abbott (U.S.), Bio-Rad Laboratories Inc. (U.S.), QIAGEN (Germany), Bayer AG (Germany), OraSure Technologies, Inc. (U.S.), Sysmex Corporation (Japan), Meridian Bioscience (U.S.), Biogate Laboratories Ltd. (Canada), J. Mitra & Co. Pvt. Ltd (India), General Biologicals Corporation (Taiwan), BioGenex (China), F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (U.S.), Siemens Healthcare GmbH (Germany), Thermo Fisher Scientific Inc. (U.S.), and Ortho Clinical Diagnostics (U.S.), Quest Diagnostics Incorporated (U.S.), Abcam Plc (U.K.) |
|
Market Opportunities |
|
Market Definition
HIV, also known as human immunodeficiency virus, is a virus that interferes with the function of cells in the body that protect against harmful diseases and infections. HIV infection is contagious and can be spread through contact with infected blood, sperm, or vaginal fluids. Human immunodeficiency virus (HIV) is thus a medical technology used to determine whether a person is HIV positive or negative.
Human Immunodeficiency Virus (HIV) Diagnosis Market Dynamics
Drivers
- Several developments and improved HIV diagnostics test kits
The growing awareness and accessibility of improved HIV diagnostics kits, especially in the low and middle-income countries will propel the HIV diagnostics market statistics. Furthermore, several regulatory authorities approving advanced products to combat the high HIV disease burden will stimulate overall market growth. Furthermore, several market participants are heavily investing in R&D activities for the development of advanced HIV diagnostic kits, which will boost the industry's growth potential. With rising HIV prevalence rates and a surge in demand for HIV diagnostic tests in underdeveloped and developing countries, the market is expected to see lucrative growth opportunities diminish during the forecast period.
- Growing repetitive usage of viral load testing in HIV patients with ART
A viral load test is a laboratory test that is used to determine the viral load in a patient's body. The term "electronic commerce" refers to the sale of electronic goods. High usage of viral load test is performed repeatedly to monitor viral load in the patients undergoing ART treatment. These tests can now be performed outside of laboratories using advanced point-of-care molecular testing. Furthermore, the presence of key industry players in the segment and a strong product portfolio will drive segmental demand. For instance, in 2019, Roche Diagnostics launched Cobas plasma separation card for viral load testing. This has helped to expand the company's HIV diagnostics product portfolio. Thus, several developments in the segment will boost the adoption of viral load tests.
Opportunities
- Increase in popularity of home-use Human immunodeficiency virus (HIV) devices
The USFDA has recently approved a few home-based diagnosis kits that can provide an HIV test result in 20 to 40 minutes without sending the test sample to a laboratory. Home-based diagnostic devices are gaining popularity in countries such as the United States and the United Kingdom over standard laboratory-use devices because they are more convenient to use, cost less, and save time. Many people are hesitant to visit their doctor or a healthcare facility to be tested for AIDS, even if they have been engaging in behaviour that puts them at risk of infection. One of the reasons for their reluctance is the social stigma associated with HIV.
Restraints/Challenges
- High cost
The high costs associated with the Human immunodeficiency virus (HIV) technological system, will hamper the market growth rate. A shortage of skilled medical professionals will also hamper the Human immunodeficiency virus (HIV) diagnosis market.
This human immunodeficiency virus (HIV) diagnosis market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the human immunodeficiency virus (HIV) diagnosis market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on the Human Immunodeficiency Virus (HIV) Diagnosis Market
The COVID-19 outbreak adversely impacted on human immunodeficiency virus (HIV) diagnosis market in 2020. Many parts of the world implemented preventive lockdown to combat community transmission of the COVID-19 virus. This has resulted in a significant decrease in the number of HIV diagnostic procedures and patient visits in healthcare facilities, negatively impacting market growth. However, with a decrease in the number of COVID-19 cases, the market is expected to grow significantly in 2021. The implementation of standard operating protocols in healthcare facilities, as well as a lower mortality rate associated with COVID-19, has resulted in an increase in elective procedures in settings with a lower risk of infection. As a result of declining COVID 19 patients, the market for 19 patients is expected to grow significantly in the near future.
Recent Developments
- In 2020, Atomo Diagnostic signed an HIV self-test distribution agreement with PrEP Health Pty Ltd. This has expanded the customer base and strengthened the HIV diagnostics product portfolio.
- In 2020, Hologic Inc. received FDA approval for its HIV-1 viral load monitoring assay. This has aided the company's efforts to broaden its product portfolio in the HIV diagnostics market.
- In 2020, OraSure Technologies, Inc. acquired UrSure, Inc., a well-known laboratory testing company. This acquisition has expanded the company's HIV diagnostic product offerings.
Global Human Immunodeficiency Virus (HIV) Diagnosis Market Scope
The human immunodeficiency virus (HIV) diagnosis market is segmented on the basis of product type, test type and application. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Consumables
- Assay
- Kits and Reagents
- Other Consumables
- Instruments
- Software
- Services
Test Type
- Antibody Tests
HIV-1 Screening Tests
- ELISA
- Rapid Tests
- Home Access Dried Blood Spot
HIV-1 Confirmatory Tests
- Western Blot Tests
- Indirect Immune Fluorescent Antibody Assays (IFA)
- Line Immunoassays (LIA)
- Radio-Immune-Precipitation Assays (RIPA)
HIV-2
Group-o-Diagnostic Tests
- CD4 Count
- Viral Load
- Early Infant
- Viral Identification
Application
- Diagnostic Laboratories
- Hospitals
- Blood Banks
- Home Care Settings
- Others
Human Immunodeficiency Virus (HIV) Diagnosis Market Regional Analysis/Insights
The human immunodeficiency virus (HIV) diagnosis market is analyzed and market size insights and trends are provided by country, product type, test type and application as referenced above.
The countries covered in the human immunodeficiency virus (HIV) diagnosis market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the human immunodeficiency virus (HIV) diagnosis market due to the increased need for effective healthcare services brought on by the high prevalence of HIV infection and expanding healthcare infrastructure.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 owing to the growing acceptance of HIV self-testing kits.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The human immunodeficiency virus (HIV) diagnosis market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for Human immunodeficiency virus (HIV) market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the human immunodeficiency virus (HIV) diagnosis market. The data is available for historic period 2011-2021.
Competitive Landscape and Human Immunodeficiency Virus (HIV) Diagnosis Market Share Analysis
The human immunodeficiency virus (HIV) diagnosis market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to human immunodeficiency virus (HIV) diagnosis market.
Some of the major players operating in the human immunodeficiency virus (HIV) diagnosis market are:
- Abbott (U.S.)
- Bio-Rad Laboratories Inc. (U.S.)
- QIAGEN (Germany)
- Bayer AG (Germany)
- OraSure Technologies, Inc. (U.S.)
- Sysmex Corporation (Japan)
- Meridian Bioscience (U.S.)
- Biogate Laboratories Ltd. (Canada)
- J. Mitra & Co. Pvt. Ltd (India)
- General Biologicals Corporation (Taiwan)
- BioGenex (China)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Mylan N.V. (U.S.)
- Siemens Healthcare GmbH (Germany)
- Thermo Fisher Scientific Inc. (U.S.)
- Ortho Clinical Diagnostics (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Abcam Plc (U.K.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.