Global Human Rabies Vaccines Market
Market Size in USD Million
CAGR :
% 
 USD
963.53 Million
USD
1,391.36 Million
2022
2030
USD
963.53 Million
USD
1,391.36 Million
2022
2030
| 2023 –2030 | |
| USD 963.53 Million | |
| USD 1,391.36 Million | |
|
|
|
|
Human Rabies Vaccines Market Analysis and Size
Increasing global incidences of mortality because of rabies, the increasing issue of stray dogs in countries with low economic development are projected to drive the market over the predicted years. According to WHO, rabies cause fatalities approximately 10,000 people every year worldwide and almost 99% cases are because of dog bites. According to World Health Organization, rabies caused 59,000 deaths annually in 150 countries with 95% cases in rural districts in 2017.
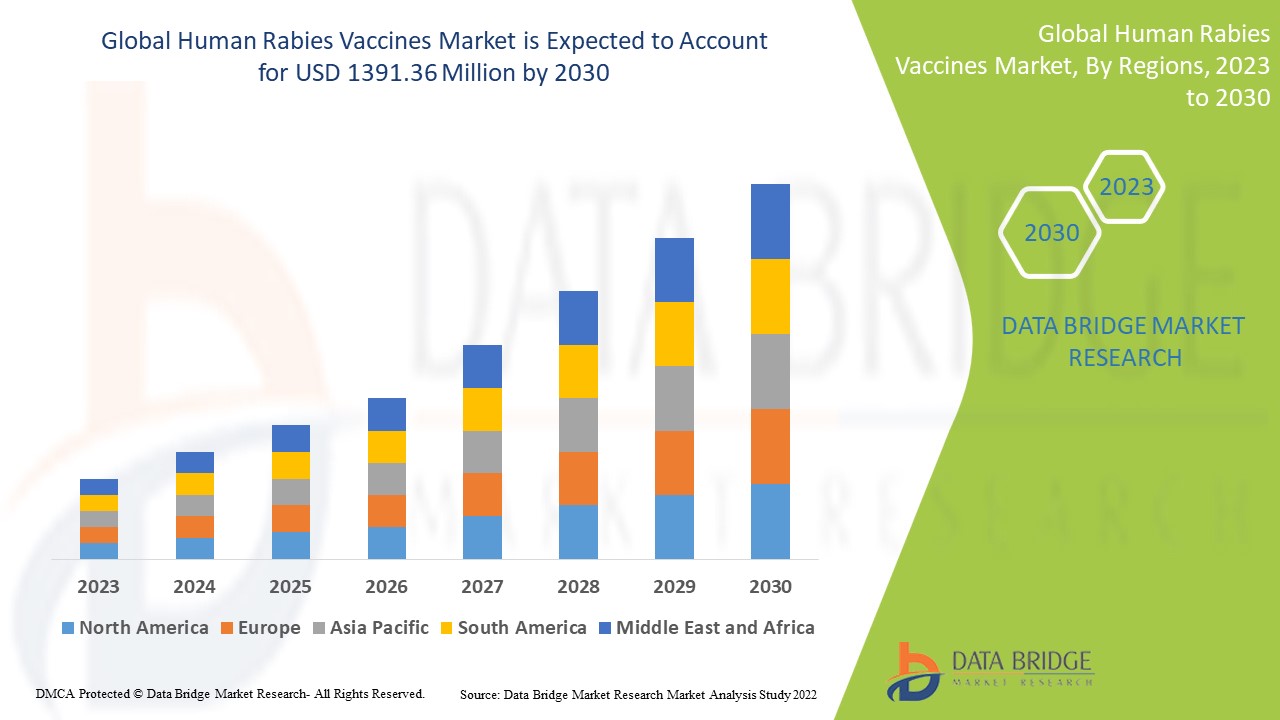
Data Bridge Market Research analyses a growth rate in the human rabies vaccines market in the forecast period 2023-2030. The expected CAGR of the human rabies vaccines market tends to be around 4.7% in the mentioned forecast period. The market was valued at USD 963.53 million in 2022 and would grow to USD 1391.36 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Human Rabies Vaccines Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021(Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Cell Line Type (Chick Embryo Cells, Vero Cell, BHK Cells, and Other), Application (Pre Exposure Prophylaxis and Post Prophylaxis), Route of Administration (Intravenous and Subcutaneous), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Serum Institute of India Pvt. Ltd (India), Novartis AG (Switzerland), Cadila Pharmaceuticals (India), and Merck & Co., Inc. (U.S.), Sanofi (France), ADS Diagnostic Ltd (India), GSK plc (U.K.), Berna Biotech Pharma GmbH (Switzerland), and AstraZeneca (U.K.) |
|
Market Opportunities |
|
Market Definition
Rabies is typically characterized as a severe disease that is caused by the rabies virus. The humans are most likely to be infected through animal bites such as dogs, cats, bats, and other mammals. The central nervous system is affected by the rabies virus which causes severe impact to the brain and may ultimately cause death in serious cases. The major symptoms include anxiety, hallucination, insomnia, hydrophobia, paralysis hyper salivation, and others. Henceforth, early prevention from the rabies is vital with the help of vaccines.
Global Human Rabies Vaccines Market Dynamics
Drivers
- Rise In The Incidence Of Rabies
The incidence of rabies is leading to a growth in the vaccine production which boosts the growth of the market. For instance, in the U.S., rabies from early 1960 to 2000 was insignificant, and a sudden rise resulted in 100 cases yearly. Although the fatalities were rare, lack of awareness towards rabies is proving to be a huge risk. From 1960 to 2018, 127 cases of rabies were stated in U.S. from dog bites and bat bites. However in Asia, rabies is a major burden, resulting for almost 35,000 deaths, fatalities caused by rabies in India are 55%.
- Increasing Technological Developments of Vaccines
There are various technological developments that are associated with the market which boost the growth of the market. For instance, in February 2018, BioNote, Inc., a South Korean veterinary diagnostic company that is operating in rabies diagnostics, announced the launch of a new immune-fluorescent device, Vcheck, that can measure diagnostic result values along with the help of fluorescent materials that helps in detecting the zoonotic disease in animals. Thus, these factors are expected to fuel the market growth in the coming years.
Opportunities
- Growing R&D Activities
Several market players are focused on research and development strategies that are expected to boost the market growth during the forecast period. For instance, in 2017, CPL Laboratories (India) which is a joint venture biotechnology company established in 2009 by Novavax Inc., USA and Cadila Pharmaceuticals Limited, stated that Nano-particle vaccine of baculvirous-derived glycoprotein which is a four dose regime vaccine is in phase-3 human trial, and is projected to be approved by 2021.
- Increasing Healthcare Expenditure
Growing healthcare expenditure by several organizations lead to the market expansion. For instance, in April 2022, the disease prevention and control bureau, Department of Health, Philippines, awarded Metro Drugs Inc., a procurement contract for 2,500,000 doses of purified chick embryo cell rabies vaccine. The total approved budget for this contract is 200,000,000 PHP.
Restraints/Challenges
- High costs will derail the market growth
The huge expenditure associated with these agents impedes the market growth. For instance, as per World Health Organization, in 2017, the projected cost of PEP was US $1.5 million, which can make it too expensive in mid- or low-income countries and limit the growth of the market during the forecast period.
This human rabies vaccines market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the human rabies vaccines market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Human Rabies Vaccines Market
The COVID-19 pandemic had a negative impact on the market. The pandemic impacted every aspect of veterinary healthcare, which includes vaccinations. For instance, according to the study titled, "The urgency of resuming disrupted dog rabies vaccination campaigns: a modeling and cost-effectiveness analysis," published in June 2021, Haiti’s 2020 dog vaccination campaign was canceled, and funds were shifted to support the COVID-19 response. According to 2020 data from the Centers for Disease Control and Prevention (CDC), cats, dogs, and several other species were at higher risk of contracting the virus. Thus, the initial impact of COVID-19 on the market growth was severe due to lockdowns, virus curbing measures, and overall negligence toward the animal sector, though such developments are estimated to boost the market growth during the coming years.
Recent Development
- In April 2022, Cadila Pharmaceuticals launched a three-dose recombinant nanoparticle-based G protein rabies vaccine in Ahmedabad. This regimen is to be given over one week (on day 0, day three, and day seven) after exposure to the virus. It is the first in a long list of existing rabies vaccines that require five doses.
Global Human Rabies Vaccines Market Scope
The human rabies vaccines market is segmented on the basis of cell line type, application, route of administration, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Cell Line Type
- Chick Embryo Cells
- Vero Cell
- BHK Cells
- Other
Application
- Pre Exposure Prophylaxis
- Post Prophylaxis
Route of Administration
- Intravenous
- Subcutaneous
End User
- Hospitals
- Homecare
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Human Rabies Vaccines Market Regional Analysis/Insights
The human rabies vaccines market is analyzed and market size insights and trends are provided by cell line type, application, route of administration, distribution channel and end-user as referenced above.
The major countries covered in the human rabies vaccines market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Asia-Pacific has been witnessing positive growth for the human rabies vaccines market throughout the forecasted period due to the presence of key manufacturers of the product is high and, increasing research and development activities, healthcare expenditure.
North America dominates the market due to major product launches, high concentration of market players and manufacturers' presence, numerous acquisitions and partnerships among major players, and government initiatives toward rabies vaccination.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Human Rabies Vaccines Market Share Analysis
The human rabies vaccines market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to human rabies vaccines market.
Key players operating in the human rabies vaccines market include:
- Serum Institute of India Pvt. Ltd (India)
- Novartis AG (Switzerland)
- Cadila Pharmaceuticals (India)
- Merck & Co., Inc. (U.S.)
- Sanofi (France)
- ADS Diagnostic Ltd (India)
- GSK plc (U.K.)
- Berna Biotech Pharma GmbH (Switzerland)
- AstraZeneca (U.K.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL HUMAN RABIES VACCINES MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL HUMAN RABIES VACCINES MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 PIPELINE CURVE
2.2.3 MARKET GUIDE
2.2.4 COMPANY POSITIONING GRID
2.2.5 COMPANY MARKET SHARE ANALYSIS
2.2.6 MULTIVARIATE MODELLING
2.2.7 TOP TO BOTTOM ANALYSIS
2.2.8 STANDARDS OF MEASUREMENT
2.2.9 VENDOR SHARE ANALYSIS
2.2.10 EPIDEMIOLOGY MODELLING
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL HUMAN RABIES VACCINES MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S 5 FORCES
6 EPIDEMIOLOGY
7 INDUSTRY INSIGHTS
8 REGULATORY FRAMEWORK
9 PIPELINE ANALYSIS
9.1 PHASE III CANDIDATES
9.2 PHASE II CANDIDATES
9.3 PHASE I CANDIDATES
9.4 OTHERS (PRE-CLINICAL AND RESEARCH)
10 GLOBAL HUMAN RABIES VACCINES MARKET, BY CELL LINE TYPE
10.1 OVERVIEW
10.2 MARKETED
10.2.1 HUMAN DIPLOID CELL VACCINE (HDCV)
10.2.2 PURIFIED CHICK EMBRYO CELL VACCINE (PCECV)
10.2.3 PURIFIED VERO CELL VACCINE (PVRV)
10.2.4 BABY HAMSTER KIDNEY (BHK) CELLS
10.2.5 OTHER
10.3 PIPLINE/EMERGING
10.3.1 PROTEIN VACCINES
10.3.2 ADJUVANTED RABIES VACCINES
10.3.3 GENETIC VACCINES
10.3.4 RNA VACCINES
10.3.5 DNA VACCINES
10.3.6 VIRAL VECTOR VACCINES
11 GLOBAL HUMAN RABIES VACCINES MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 PRE EXPOSURE PROPHYLAXIS
11.3 POST EXPOSURE PROPHYLAXIS
12 GLOBAL HUMAN RABIES VACCINES MARKET, BY ROUTE OF ADMINISTRATION
12.1 OVERVIEW
12.2 INTRAMUSCULAR
12.3 INTRADERMAL
13 GLOBAL HUMAN RABIES VACCINES MARKET, BY BRAND
13.1 OVERVIEW
13.2 IMOVAX RABIES
13.3 RABAVERT
13.4 ABHAYRAB
13.5 BERIRAB-P
13.6 CARIG
13.7 EQUIRAB
13.8 OTHER
14 GLOBAL HUMAN RABIES VACCINES MARKET, BY POPULATION TYPE
14.1 OVERVIEW
14.2 PEDIATRIC
14.3 ADULT
15 GLOBAL HUMAN RABIES VACCINES MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITALS
15.2.1 PRIVATE
15.2.2 PUBLIC
15.3 SPECIALITY CLINICS
15.4 COMMUNITY CENTER
15.5 OTHER
16 GLOBAL HUMAN RABIES VACCINES MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDERS
16.3 RETAILS PHARMACIES
16.4 ONLINE PHARMACIES
16.5 OTHERS
17 GLOBAL HUMAN RABIES VACCINES MARKET, BY GEOGRAPHY
17.1 GLOBAL HUMAN RABIES VACCINES MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
17.2 NORTH AMERICA
17.2.1 U.S.
17.2.2 CANADA
17.2.3 MEXICO
17.3 EUROPE
17.3.1 GERMANY
17.3.2 U.K.
17.3.3 ITALY
17.3.4 FRANCE
17.3.5 SPAIN
17.3.6 RUSSIA
17.3.7 SWITZERLAND
17.3.8 TURKEY
17.3.9 BELGIUM
17.3.10 NETHERLANDS
17.3.11 DENMARK
17.3.12 SWEDEN
17.3.13 POLAND
17.3.14 NORWAY
17.3.15 FINLAND
17.3.16 REST OF EUROPE
17.4 ASIA-PACIFIC
17.4.1 JAPAN
17.4.2 CHINA
17.4.3 SOUTH KOREA
17.4.4 INDIA
17.4.5 SINGAPORE
17.4.6 THAILAND
17.4.7 INDONESIA
17.4.8 MALAYSIA
17.4.9 PHILIPPINES
17.4.10 AUSTRALIA
17.4.11 NEW ZEALAND
17.4.12 VIETNAM
17.4.13 TAIWAN
17.4.14 REST OF ASIA-PACIFIC
17.5 SOUTH AMERICA
17.5.1 BRAZIL
17.5.2 ARGENTINA
17.5.3 REST OF SOUTH AMERICA
17.6 MIDDLE EAST AND AFRICA
17.6.1 SOUTH AFRICA
17.6.2 EGYPT
17.6.3 BAHRAIN
17.6.4 UNITED ARAB EMIRATES
17.6.5 KUWAIT
17.6.6 OMAN
17.6.7 QATAR
17.6.8 SAUDI ARABIA
17.6.9 REST OF MEA
18 COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: GLOBAL
18.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
18.3 COMPANY SHARE ANALYSIS: EUROPE
18.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
18.5 MERGERS & ACQUISITIONS
18.6 NEW PRODUCT DEVELOPMENT & APPROVALS
18.7 EXPANSIONS
18.8 REGULATORY CHANGES
18.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
19 GLOBAL HUMAN RABIES VACCINES MARKET, SWOT AND DBMR ANALYSIS
20 GLOBAL HUMAN RABIES VACCINES MARKET, COMPANY PROFILE
20.1 BHARAT BIOTECH
20.1.1 COMPANY OVERVIEW
20.1.2 REVENUE ANALYSIS
20.1.3 GEOGRAPHIC PRESENCE
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.2 SERUM INSTITUTE OF INDIA PVT. LTD.
20.2.1 COMPANY OVERVIEW
20.2.2 REVENUE ANALYSIS
20.2.3 GEOGRAPHIC PRESENCE
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 NOVARTIS AG
20.3.1 COMPANY OVERVIEW
20.3.2 REVENUE ANALYSIS
20.3.3 GEOGRAPHIC PRESENCE
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENTS
20.4 CADILA PHARMACEUTICALS.
20.4.1 COMPANY OVERVIEW
20.4.2 REVENUE ANALYSIS
20.4.3 GEOGRAPHIC PRESENCE
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENTS
20.5 MERCK & CO., INC.
20.5.1 COMPANY OVERVIEW
20.5.2 REVENUE ANALYSIS
20.5.3 GEOGRAPHIC PRESENCE
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENTS
20.6 SANOFI
20.6.1 COMPANY OVERVIEW
20.6.2 REVENUE ANALYSIS
20.6.3 GEOGRAPHIC PRESENCE
20.6.4 PRODUCT PORTFOLIO
20.6.5 RECENT DEVELOPMENTS
20.7 GSK PLC
20.7.1 COMPANY OVERVIEW
20.7.2 REVENUE ANALYSIS
20.7.3 GEOGRAPHIC PRESENCE
20.7.4 PRODUCT PORTFOLIO
20.7.5 RECENT DEVELOPMENTS
20.8 BERNA BIOTECH PHARMA GMBH
20.8.1 COMPANY OVERVIEW
20.8.2 REVENUE ANALYSIS
20.8.3 GEOGRAPHIC PRESENCE
20.8.4 PRODUCT PORTFOLIO
20.8.5 RECENT DEVELOPMENTS
20.9 BOEHRINGER INGELHEIM INTERNATIONAL GMBH.
20.9.1 COMPANY OVERVIEW
20.9.2 REVENUE ANALYSIS
20.9.3 GEOGRAPHIC PRESENCE
20.9.4 PRODUCT PORTFOLIO
20.9.5 RECENT DEVELOPMENTS
20.1 BIO-MED
20.10.1 COMPANY OVERVIEW
20.10.2 REVENUE ANALYSIS
20.10.3 GEOGRAPHIC PRESENCE
20.10.4 PRODUCT PORTFOLIO
20.10.5 RECENT DEVELOPMENTS
20.11 INDIAN IMMUNOLOGICALS LTD
20.11.1 COMPANY OVERVIEW
20.11.2 REVENUE ANALYSIS
20.11.3 GEOGRAPHIC PRESENCE
20.11.4 PRODUCT PORTFOLIO
20.11.5 RECENT DEVELOPMENTS
20.12 KEDRION BIOPHARMA
20.12.1 COMPANY OVERVIEW
20.12.2 REVENUE ANALYSIS
20.12.3 GEOGRAPHIC PRESENCE
20.12.4 PRODUCT PORTFOLIO
20.12.5 RECENT DEVELOPMENTS
20.13 GRIFOLS, S.A.
20.13.1 COMPANY OVERVIEW
20.13.2 REVENUE ANALYSIS
20.13.3 GEOGRAPHIC PRESENCE
20.13.4 PRODUCT PORTFOLIO
20.13.5 RECENT DEVELOPMENTS
20.14 BAVARIAN NORDIC
20.14.1 COMPANY OVERVIEW
20.14.2 REVENUE ANALYSIS
20.14.3 GEOGRAPHIC PRESENCE
20.14.4 PRODUCT PORTFOLIO
20.14.5 RECENT DEVELOPMENTS
20.15 KAMADA PHARMACEUTICALS
20.15.1 COMPANY OVERVIEW
20.15.2 REVENUE ANALYSIS
20.15.3 GEOGRAPHIC PRESENCE
20.15.4 PRODUCT PORTFOLIO
20.15.5 RECENT DEVELOPMENTS
20.16 CHIRON BEHRING PVT. LTD.
20.16.1 COMPANY OVERVIEW
20.16.2 REVENUE ANALYSIS
20.16.3 GEOGRAPHIC PRESENCE
20.16.4 PRODUCT PORTFOLIO
20.16.5 RECENT DEVELOPMENTS
20.17 ZUVENTUS HEALTHCARE LTD
20.17.1 COMPANY OVERVIEW
20.17.2 REVENUE ANALYSIS
20.17.3 GEOGRAPHIC PRESENCE
20.17.4 PRODUCT PORTFOLIO
20.17.5 RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
21 RELATED REPORTS
22 CONCLUSION
23 QUESTIONNAIRE
24 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












