Global Subcutaneous Neurofibromas Market
Market Size in USD Billion
CAGR :
% 
 USD
12.72 Billion
USD
20.27 Billion
2024
2032
USD
12.72 Billion
USD
20.27 Billion
2024
2032
| 2025 –2032 | |
| USD 12.72 Billion | |
| USD 20.27 Billion | |
|
|
|
|
Subcutaneous Neurofibromas Market Size
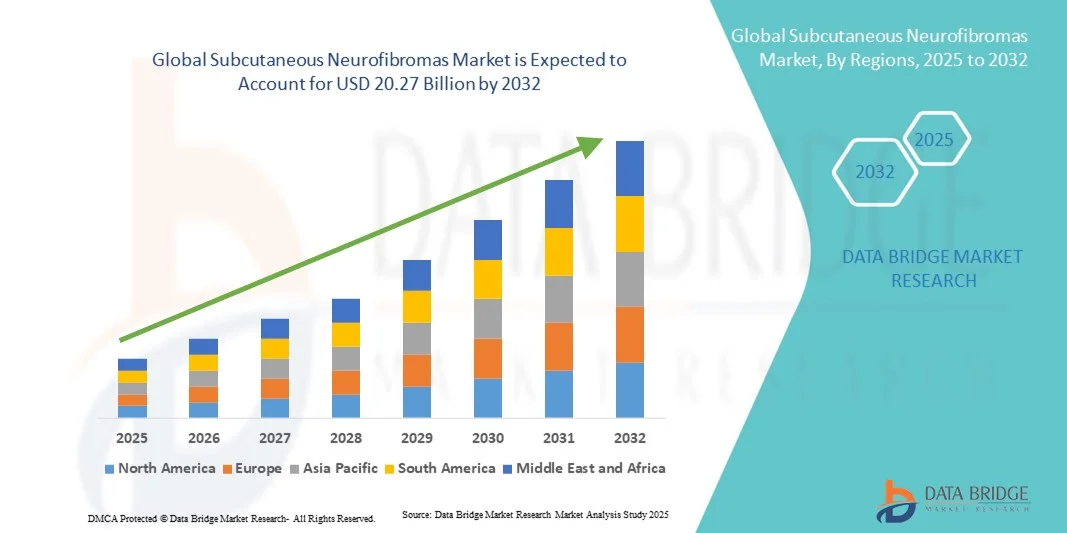
- The global subcutaneous neurofibromas market size was valued at USD 12.72 billion in 2024 and is expected to reach USD 20.27 billion by 2032, at a CAGR of 6.00% during the forecast period
- The market growth is largely fuelled by rising incidence of nerve‑tumour conditions such as neurofibromas, increased clinical trials and approvals of treatment modalities, and improvements in diagnostic imaging and surgical interventions
- Furthermore, growing awareness among patients and healthcare providers about minimally invasive therapies, targeted therapies and advanced diagnostics are establishing subcutaneous neurofibromas management as a specialized niche within neurology/oncology. These converging factors are accelerating the uptake of treatment solutions, thereby significantly boosting the industry’s growth
Subcutaneous Neurofibromas Market Analysis
- Subcutaneous neurofibromas, benign tumors arising from peripheral nerves, are increasingly recognized as a critical clinical concern in both pediatric and adult populations due to their prevalence in patients with Neurofibromatosis Type 1 (NF1) and potential impact on quality of life
- The escalating demand for subcutaneous neurofibroma management is primarily fueled by rising awareness of NF1, advancements in diagnostic imaging techniques, and the development of targeted therapies such as MEK inhibitors, enabling more effective and personalized treatment approaches
- North America dominated the subcutaneous neurofibromas market with the largest revenue share of 38.4% in 2024, driven by advanced healthcare infrastructure, high patient awareness, presence of key treatment and diagnostic players, and increasing adoption of novel therapies and minimally invasive surgical procedures
- Asia-Pacific is expected to be the fastest-growing region in the subcutaneous neurofibromas market during the forecast period due to rising diagnosis rates, growing healthcare investments, expanding access to specialized treatment centers, and increased availability of advanced distribution channels, including hospital and online pharmacies
- Surgery segment dominated the subcutaneous neurofibromas market with a market share of 42.9% in 2024, supported by its established clinical efficacy, while MEK inhibitors are witnessing rapid adoption as emerging targeted therapies complementing traditional interventions
Report Scope and Subcutaneous Neurofibromas Market Segmentation
|
Attributes |
Subcutaneous Neurofibromas Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Subcutaneous Neurofibromas Market Trends
Integration of Targeted Therapies and Minimally Invasive Techniques
- A significant and accelerating trend in the global subcutaneous neurofibromas market is the increasing adoption of targeted therapies such as MEK inhibitors combined with minimally invasive surgical techniques, enhancing treatment efficacy and patient outcomes
- For instance, Selumetinib, a MEK inhibitor approved for pediatric NF1 patients with inoperable plexiform neurofibromas, is being used alongside precision excision procedures to reduce tumor size and improve quality of life
- Integration of genetic profiling and advanced imaging allows clinicians to personalize treatment plans, predict disease progression, and monitor therapy response with greater accuracy
- For instance, high-resolution MRI and electromyography are being used to map tumor location and guide surgical excision, minimizing damage to surrounding nerves
- This trend toward precision medicine and combination therapy is reshaping treatment protocols, encouraging development of novel therapeutics and improving patient adherence and outcomes
- The demand for advanced, targeted, and minimally invasive treatment options is growing rapidly across pediatric and adult NF1 populations, as patients and clinicians increasingly prioritize effectiveness, safety, and reduced recovery times
Subcutaneous Neurofibromas Market Dynamics
Driver
Rising Prevalence of NF1 and Awareness of Subcutaneous Tumors
- The increasing prevalence of Neurofibromatosis Type 1 and growing awareness of subcutaneous neurofibromas are key drivers for the heightened demand for diagnosis and treatment
- For instance, in 2024, specialized NF1 clinics in North America and Europe reported increasing patient enrollment and earlier diagnosis through genetic screening programs
- As patients and caregivers become more aware of available treatment options, demand for surgical excision, MEK inhibitors, and imaging-guided interventions is rising. For instance, hospitals and diagnostic centers are incorporating combined therapy protocols to manage tumor growth and associated symptoms more effectively
- The availability of integrated healthcare services, along with advancements in diagnostics and personalized therapy, further supports market growth by improving treatment outcomes and patient satisfaction
- For instance, multi-disciplinary treatment centers in Asia-Pacific are expanding NF1 programs, integrating clinical management, imaging, and therapy access to meet growing patient needs
- Rising funding and grants for NF1 research are accelerating innovation in treatment options and early diagnosis methods
- Expansion of healthcare insurance coverage for rare disease treatments is supporting patient access to advanced therapies and diagnostics
Restraint/Challenge
High Treatment Costs and Regulatory Barriers
- Concerns surrounding the high cost of targeted therapies and surgical interventions pose a significant challenge to broader market adoption, particularly in developing regions
- For instance, MEK inhibitors such as Selumetinib require ongoing monitoring and can be cost-prohibitive for many patients without insurance coverage
- Regulatory approvals for new drugs and therapies can be lengthy and complex, delaying patient access to innovative treatment options. For instance, novel MEK inhibitors in clinical trials face multi-year regulatory review before receiving market authorization in key regions such as Europe and Asia-Pacific
- The relative lack of awareness in emerging markets and limited access to specialized NF1 treatment centers further restricts widespread adoption and early intervention
- For instance, rural hospitals and clinics may lack advanced imaging equipment or trained specialists, limiting patient access to minimally invasive surgeries or targeted therapy programs
- Limited long-term clinical data on emerging therapies may create hesitation among physicians and patients to adopt new treatments
- Variability in reimbursement policies across regions can restrict patient affordability and slow market penetration
Subcutaneous Neurofibromas Market Scope
The market is segmented on the basis of treatment, diagnosis, symptoms, end-users, and distribution channel.
- By Treatment
On the basis of treatment, the subcutaneous neurofibromas market is segmented into surgery, biopsy, laser treatment, MEK inhibitors, and others. The surgery segment dominated the market in 2024, holding the largest revenue share of 42.9%, driven by its established efficacy and widespread adoption in clinical practice. Surgical excision remains the first-line treatment for accessible tumors and provides immediate relief from pain, numbness, or visible lumps. Hospitals and specialty clinics prefer surgery due to its predictability, proven outcomes, and the ability to combine it with imaging guidance for precision. Continuous innovations in minimally invasive surgical tools are reducing recovery time and improving patient satisfaction. For instance, pediatric and adult NF1 patients frequently undergo surgical excision for symptomatic or growing subcutaneous neurofibromas, boosting overall segment adoption.
The MEK inhibitors segment is expected to witness the fastest growth from 2025 to 2032, fueled by increasing adoption of targeted therapies for NF1-associated tumors. MEK inhibitors provide a non-invasive alternative to surgery, particularly for inoperable or recurrent tumors, and are showing promising efficacy in clinical trials. These therapies support long-term disease management and reduce tumor progression, making them suitable for both pediatric and adult patients. For instance, Selumetinib has gained significant traction in North America and Europe due to regulatory approvals and growing clinician awareness. Increasing clinical studies and collaborations between pharmaceutical companies and research institutions are accelerating adoption globally. Furthermore, MEK inhibitors are often combined with surgical or imaging-guided interventions to optimize patient outcomes.
- By Diagnosis
On the basis of diagnosis, the market is segmented into electromyography, MRI, CT scan, and others. The MRI segment dominated the market in 2024 due to its high accuracy in detecting nerve-sheath tumors and mapping tumor location for surgical planning. MRI is the preferred imaging modality for both diagnosis and monitoring treatment response, providing detailed soft tissue contrast without radiation exposure. Hospitals and diagnostic centers favor MRI for its reliability and ability to track tumor progression over time. MRI also integrates seamlessly with surgical planning tools and genetic profiling data, supporting precision medicine approaches. For instance, clinicians use MRI to guide minimally invasive surgery and monitor MEK inhibitor efficacy. Advanced MRI techniques allow earlier detection of smaller subcutaneous neurofibromas, improving prognosis and treatment timing.
The electromyography segment is expected to witness the fastest growth from 2025 to 2032, driven by rising adoption in specialized clinics and research centers. EMG helps assess nerve function and determine the extent of peripheral nerve involvement in patients with subcutaneous neurofibromas. For instance, EMG is increasingly used in combination with MRI to personalize treatment strategies, particularly in complex or recurrent cases. Its low cost, ease of use, and diagnostic complementarity make it an attractive choice for hospitals and diagnostic centers globally. EMG adoption is also growing due to rising clinician awareness and better insurance coverage in developed regions.
- By Symptoms
On the basis of symptoms, the market is segmented into pain, dizziness, numbness, itching, lump under the skin, and others. The lump under the skin segment dominated in 2024 with the largest share, as visible tumors often drive patients to seek early medical intervention. Subcutaneous lumps are frequently the first noticeable symptom, prompting clinical evaluation and imaging, which boosts diagnosis rates. The segment also benefits from patient awareness campaigns and educational programs highlighting NF1 symptoms. For instance, patients reporting lumps are often referred to specialized NF1 clinics for treatment planning, increasing hospital and clinic adoption of treatment protocols. Lumps often coincide with other symptoms, reinforcing early diagnosis and treatment. Regular monitoring of visible lumps helps in predicting tumor growth and deciding between surgical or targeted interventions.
The pain segment is expected to witness the fastest growth from 2025 to 2032, driven by increasing focus on symptom management and quality-of-life improvements. Painful tumors prompt earlier intervention through surgery or targeted therapy. For instance, hospitals and clinics are adopting multimodal approaches combining analgesics, surgery, and MEK inhibitors to manage pain effectively. Growing patient awareness of available treatments is further accelerating market uptake in this segment. Chronic pain management programs in NF1 specialty centers are also boosting demand for combination therapies. Pain reporting apps and telemedicine monitoring are contributing to higher patient engagement and adherence.
- By End-Users
On the basis of end-users, the market is segmented into clinics, hospitals, diagnostic centers, and others. The hospital segment dominated in 2024 due to the availability of multidisciplinary teams, advanced imaging equipment, and surgical facilities capable of managing complex NF1 cases. Hospitals also offer integrated treatment plans combining surgery, targeted therapy, and follow-up care, ensuring better patient outcomes. For instance, major hospitals in North America and Europe provide comprehensive NF1 programs that include treatment, genetic counseling, and long-term monitoring. Hospitals maintain specialist staff for personalized care, enhancing patient trust. Hospitals also serve as key sites for clinical trials and adoption of novel therapies, further driving revenue share.
The diagnostic center segment is expected to witness the fastest growth from 2025 to 2032, driven by the rising number of standalone diagnostic centers offering MRI, EMG, and CT scan services. For instance, specialized centers in Asia-Pacific are expanding capabilities for early NF1 detection and treatment monitoring. Increased awareness and accessibility of advanced diagnostic techniques are facilitating market growth in these centers. Diagnostic centers also support telemedicine and remote patient monitoring, increasing efficiency. Their cost-effectiveness and focus on early diagnosis make them attractive to patients and healthcare providers asuch as.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated in 2024 due to direct availability of therapies such as MEK inhibitors and post-surgical medications within hospital settings, ensuring continuity of care and proper dosage supervision. Hospitals also manage patient adherence and monitoring, particularly for chronic or long-term therapies. For instance, hospital pharmacies in North America provide Selumetinib and supportive medications for NF1 patients following clinical guidelines. Hospitals coordinate therapy distribution with ongoing patient follow-up, improving outcomes. Integration with hospital EMRs allows accurate prescription tracking and treatment optimization.
The online pharmacy segment is expected to witness the fastest growth from 2025 to 2032, driven by increasing adoption of telemedicine, e-prescriptions, and digital health solutions. For instance, patients in remote regions can access targeted therapies and supportive care medications via online pharmacies, improving treatment adherence and convenience. Rising internet penetration and patient preference for home delivery are further fueling growth in this channel. Online pharmacies also offer access to educational resources, reminders, and teleconsultation support. E-commerce platforms for rare disease medications are expanding globally, enhancing market penetration.
Subcutaneous Neurofibromas Market Regional Analysis
- North America dominated the subcutaneous neurofibromas market with the largest revenue share of 38.4% in 2024, driven by advanced healthcare infrastructure, high patient awareness, presence of key treatment and diagnostic players, and increasing adoption of novel therapies and minimally invasive surgical procedures
- Patients and clinicians in the region prioritize early diagnosis and effective management of NF1-associated subcutaneous neurofibromas, leveraging advanced imaging techniques such as MRI and electromyography alongside surgical and targeted therapies such as MEK inhibitors
- This widespread adoption is further supported by well-established NF1 specialty clinics, integrated treatment programs, and high healthcare expenditure, enabling comprehensive patient care
U.S. Subcutaneous Neurofibromas Market Insight
The U.S. subcutaneous neurofibromas market captured the largest revenue share of ~40% in 2024 within North America, fueled by rising awareness of NF1 and increasing access to advanced treatment options. Patients and clinicians are prioritizing early diagnosis through MRI, CT scans, and electromyography to enable timely intervention. The growing adoption of targeted therapies such as MEK inhibitors, combined with minimally invasive surgical procedures, is further propelling the market. Moreover, well-established NF1 specialty clinics and multi-disciplinary hospital programs are providing integrated care, including genetic counseling, monitoring, and therapy management. Increasing insurance coverage and reimbursement for rare disease treatments are enhancing patient access to advanced therapies. Furthermore, ongoing clinical trials and collaborations between pharmaceutical companies and research institutions are accelerating the availability of innovative treatment solutions.
Europe Subcutaneous Neurofibromas Market Insight
The Europe subcutaneous neurofibromas market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by growing NF1 awareness and adoption of advanced diagnostic techniques. The increasing prevalence of subcutaneous neurofibromas, combined with government initiatives supporting rare disease management, is encouraging early detection and treatment. Hospitals and diagnostic centers across the region are integrating advanced imaging, genetic testing, and personalized treatment protocols. European patients are also drawn to minimally invasive surgical approaches and targeted therapies, which improve outcomes and reduce recovery times. The market is witnessing growth across both adult and pediatric NF1 populations, with specialized centers enhancing accessibility to treatment. Moreover, cross-border collaborations in clinical research are fostering the introduction of novel therapies in the region.
U.K. Subcutaneous Neurofibromas Market Insight
The U.K. subcutaneous neurofibromas market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising NF1 awareness and demand for comprehensive care. Concerns regarding disease progression, visible lumps, and related pain are prompting patients to seek early medical intervention. Hospitals, clinics, and diagnostic centers are increasingly adopting advanced imaging and genetic profiling for accurate diagnosis and treatment planning. The U.K.’s healthcare infrastructure, coupled with well-established NF1 specialty programs, supports access to surgical and targeted therapies. Increasing funding for rare disease research and patient assistance programs further strengthens market growth. Moreover, the adoption of telemedicine and online pharmacies is enhancing patient monitoring, access to medications, and follow-up care.
Germany Subcutaneous Neurofibromas Market Insight
The Germany subcutaneous neurofibromas market is expected to expand at a considerable CAGR during the forecast period, fueled by advanced healthcare infrastructure and patient awareness. German patients are increasingly seeking early diagnosis and personalized treatment strategies, supported by high-quality imaging, genetic testing, and multidisciplinary care teams. Hospitals and specialized clinics emphasize minimally invasive surgery and targeted therapies to manage NF1-related tumors effectively. The country’s focus on innovation and research is driving clinical trials and development of novel therapies. Moreover, robust insurance coverage and reimbursement policies improve access to advanced treatment options. Integration of telemedicine for monitoring and follow-up care is also becoming increasingly prevalent, facilitating better patient management.
Asia-Pacific Subcutaneous Neurofibromas Market Insight
The Asia-Pacific subcutaneous neurofibromas market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by rising NF1 awareness, improving healthcare infrastructure, and increasing diagnosis rates. Countries such as China, Japan, and India are witnessing growing adoption of advanced imaging technologies, minimally invasive surgical techniques, and targeted therapies. Government initiatives supporting rare disease treatment and healthcare digitalization are promoting patient access to specialized care. Moreover, the expansion of hospital networks, diagnostic centers, and online pharmacy channels is enhancing treatment availability across the region. The market growth is further supported by the rising number of clinical studies and collaborations between pharmaceutical companies and healthcare institutions. Patients in urban centers increasingly prioritize early detection and comprehensive treatment, further fueling demand.
Japan Subcutaneous Neurofibromas Market Insight
The Japan subcutaneous neurofibromas market is gaining momentum due to rising NF1 awareness, technological advancements, and a focus on patient-centered care. Patients and healthcare providers are adopting advanced imaging and genetic testing for accurate diagnosis and monitoring. Hospitals and specialty clinics emphasize minimally invasive surgical procedures and targeted therapy adoption, improving treatment outcomes. Integration of telemedicine and digital health tools enables remote monitoring and follow-up care for patients. Moreover, Japan’s aging population is driving demand for easy-to-use, safe, and effective treatment options. Clinical research and collaborations with pharmaceutical companies are further accelerating the availability of novel therapies in the country.
India Subcutaneous Neurofibromas Market Insight
The India subcutaneous neurofibromas market accounted for the largest revenue share in Asia-Pacific in 2024, attributed to rising NF1 awareness, improving healthcare infrastructure, and growing adoption of advanced diagnostics and therapies. Patients increasingly seek minimally invasive surgery, targeted MEK inhibitor therapies, and imaging-guided interventions. The government’s focus on rare disease management and smart healthcare initiatives is supporting early diagnosis and access to treatment. Hospitals, clinics, and diagnostic centers are expanding services for NF1 patients, enhancing overall care delivery. Moreover, increasing availability of online pharmacies and telemedicine platforms is improving access to medications and follow-up care. The presence of domestic manufacturers offering affordable treatment options is further propelling market growth.
Subcutaneous Neurofibromas Market Share
The Subcutaneous Neurofibromas industry is primarily led by well-established companies, including:
- Merck & Co., Inc. (U.S.)
- AstraZeneca (U.K.)
- SpringWorks Therapeutics, Inc. (U.S.)
- Healx (U.K.)
- NFlection Therapeutics, Inc. (U.S.)
- Fosun Pharmaceutical (China)
- Pasithea Therapeutics (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- Takeda Pharmaceutical Company Limited (Japan)
- Novartis AG (Switzerland)
- GL Pharm Tech Corporation (South Korea)
- CureAge Therapeutics (U.K.)
- Ikena Oncology (U.S.)
- Recursion Pharmaceuticals, Inc. (U.S.)
- Vivace Therapeutics, Inc. (U.S.)
- Genentech, Inc (U.S.)
- GSK plc (U.K.)
- CHIA TAI TIANQING PHARMACEUTICAL GROUP CO., LTD (China)
- Array BioPharma (U.S.)
- Mulberry Biotherapeutics (U.S.)
What are the Recent Developments in Global Subcutaneous Neurofibromas Market?
- In October 2025, the Children’s Tumor Foundation/NTAP network published that research on cutaneous neurofibromas (cNFs) is accelerating, particularly due to the high burden of visible and subcutaneous tumours in NF1
- In September 2025, the FDA approved Selumetinib (brand Koselugo) granules and capsules for pediatric NF1 patients aged 1 year and older with symptomatic, inoperable plexiform neurofibromas. U.S. Food and Drug Administration. This approval further expands the age indication downward in the NF1 treatment population, allowing earlier intervention
- In July 2025, a peer‑reviewed study published in Nature Communications shed new molecular‑level insights into cutaneous and subcutaneous neurofibromas. These insights may yield novel biomarker‑ or target‑driven strategies for subcutaneous neurofibroma treatment
- In February 2025, Mirdametinib was approved by the Food and Drug Administration (FDA) for adult and pediatric patients (2 years and older) with Neurofibromatosis Type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN) that could not be completely resected
- In February 2025, Healx (UK) announced the dosing of the first patient in its Phase 2 trial of HLX 1502, an investigational oral therapy for NF1. This development highlights emerging non MEK inhibitor therapeutic candidates for NF1 and associated neurofibromas
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.