Global Hyaluronidase Deficiency Market
Market Size in USD Billion
CAGR :
% 
 USD
1.55 Billion
USD
2.34 Billion
2024
2032
USD
1.55 Billion
USD
2.34 Billion
2024
2032
| 2025 –2032 | |
| USD 1.55 Billion | |
| USD 2.34 Billion | |
|
|
|
|
Hyaluronidase Deficiency Market Size
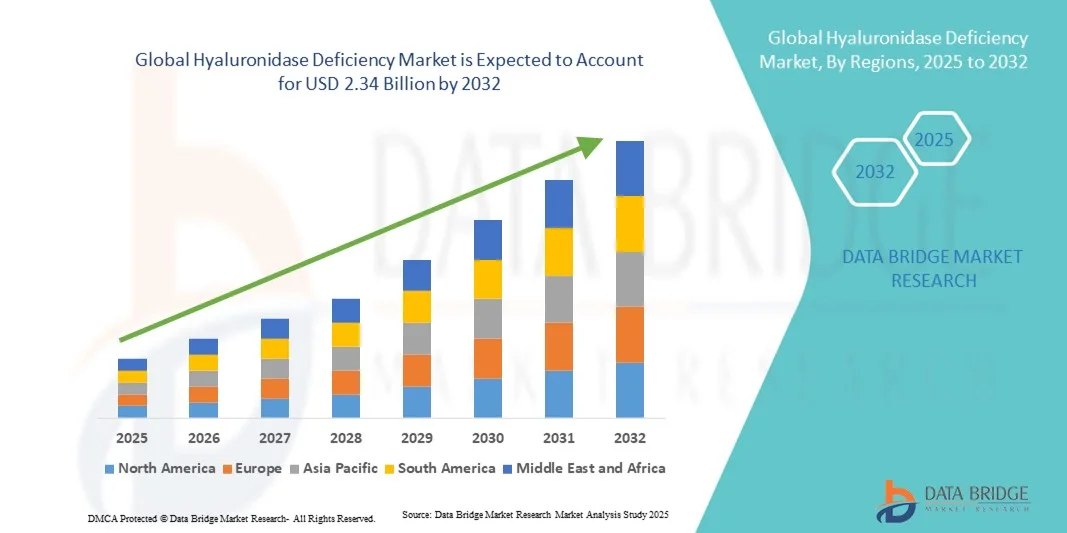
- The global hyaluronidase deficiency market size was valued at USD 1.55 billion in 2024 and is expected to reach USD 2.34 billion by 2032, at a CAGR of 5.30% during the forecast period
- The market growth is largely fueled by the growing adoption of advanced enzymatic therapies and ongoing research in rare metabolic and genetic disorders, leading to increased awareness and diagnosis of hyaluronidase deficiency across healthcare settings
- Furthermore, rising demand for effective enzyme replacement therapies (ERTs), advancements in biotechnology, and the growing focus of pharmaceutical companies on developing novel biologics are establishing Hyaluronidase Deficiency treatment as a key area of innovation. These converging factors are accelerating the uptake of hyaluronidase deficiency therapies, thereby significantly boosting the industry's growth
Hyaluronidase Deficiency Market Analysis
- Hyaluronidase Deficiency, a rare metabolic disorder characterized by the body’s inability to properly break down hyaluronic acid, has become an area of growing clinical focus due to its significant impact on connective tissue and organ function. Increasing awareness, advancements in genetic testing, and the introduction of enzyme-based and gene-targeted therapies are fueling global market expansion
- The demand for hyaluronidase deficiency treatments is primarily driven by improved diagnostic capabilities, rising government and private research funding for rare disease management, and the growing availability of orphan drug designations encouraging pharmaceutical innovation
- North America dominated the hyaluronidase deficiency market with the largest revenue share of 41.6% in 2024, supported by strong research infrastructure, well-established biopharmaceutical companies, and high awareness regarding genetic enzyme deficiencies. The U.S. remains a key contributor, driven by active clinical trials, favorable regulatory frameworks, and expanded access to rare disease therapies through specialty care programs
- Asia-Pacific is expected to be the fastest-growing region, registering a CAGR of 8.9% from 2025 to 2032, attributed to improving healthcare infrastructure, growing investment in rare disease research, and increased early diagnosis efforts in countries like Japan, China, and South Korea
- The Animal-Derived Hyaluronidase segment dominated the market with the largest revenue share of 56.4% in 2024, owing to its established clinical usage, cost efficiency, and broad therapeutic availability
Report Scope and Hyaluronidase Deficiency Market Segmentation
|
Attributes |
Hyaluronidase Deficiency Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Hyaluronidase Deficiency Market Trends
Rising Advancements in Enzyme Replacement Therapies and Precision Medicine
- A significant and accelerating trend in the global hyaluronidase deficiency market is the growing focus on advancements in enzyme replacement therapies (ERTs) and precision medicine-based approaches to effectively manage this rare metabolic disorder. Continuous R&D efforts are being directed toward developing recombinant and synthetic hyaluronidase formulations that offer enhanced enzyme stability, improved bioavailability, and prolonged therapeutic effects. These developments are transforming the treatment paradigm for patients with congenital or acquired hyaluronidase deficiency
- For instance, in March 2023, Halozyme Therapeutics, Inc. announced significant progress in its recombinant human hyaluronidase (rHuPH20) platform, expanding its clinical utility beyond oncology drug delivery enhancement into metabolic and enzymatic deficiency disorders. This reflects a broader shift towards leveraging enzyme-based biologics for targeted and sustained therapeutic benefits
- In addition, the integration of precision diagnostics and genetic sequencing technologies is facilitating early detection of enzyme deficiencies and tailoring patient-specific therapeutic regimens. For example, the adoption of next-generation sequencing (NGS) and biomarker-based diagnostics has enabled clinicians to identify enzyme mutations responsible for hyaluronidase deficiency more accurately, improving treatment outcomes and patient management strategies
- Furthermore, there is a notable trend toward the development of long-acting formulations and combination therapies that enhance enzymatic activity and reduce the frequency of administration. Biopharmaceutical companies are increasingly collaborating with academic research institutions to explore nanoparticle-based delivery systems for improved stability and targeted tissue penetration.
- This growing emphasis on innovation, patient personalization, and cross-disciplinary collaboration is reshaping the Hyaluronidase Deficiency treatment landscape. As precision medicine continues to mature and biopharmaceutical R&D investments increase, the market is expected to witness accelerated growth and diversification in therapeutic options over the next several years
Hyaluronidase Deficiency Market Dynamics
Driver
Increasing Research Investments and Expanding Clinical Applications of Hyaluronidase Enzymes
- The global Hyaluronidase Deficiency market is primarily driven by rising research investments in enzyme therapy development and expanding clinical applications of hyaluronidase-based formulations across multiple therapeutic areas. Growing awareness regarding enzyme replacement therapy as a viable treatment option for metabolic and connective tissue disorders has stimulated demand for advanced hyaluronidase products
- For instance, in April 2024, Halozyme Therapeutics announced advancements in its rHuPH20 enzyme platform, exploring its potential applications for tissue permeability enhancement and systemic enzyme deficiencies. Such strategic R&D initiatives are significantly driving the growth of the Hyaluronidase Deficiency market during the forecast period
- Moreover, the rising prevalence of enzyme deficiency-related disorders and increasing diagnostic rates due to advancements in molecular testing are contributing to the market expansion. The adoption of hyaluronidase formulations in drug delivery enhancement, dermatological procedures, and ophthalmic treatments is further broadening the scope of therapeutic applications
- Increasing collaborations between biotechnology companies and research institutions are accelerating the development of novel recombinant enzymes with enhanced efficacy and safety profiles. In addition, government and private funding for rare disease research and orphan drug development are fueling innovation in this domain
- The market growth is also supported by the emerging trend of personalized and gene-based therapies, which aim to address the underlying genetic causes of enzyme deficiencies. Continuous innovation in recombinant enzyme engineering and a strong clinical pipeline highlight the industry’s commitment to addressing unmet medical needs in hyaluronidase deficiency management
Restraint/Challenge
High Treatment Costs and Limited Accessibility in Developing Regions
- Despite the growing advancements in therapeutic innovation, the high cost of enzyme replacement therapies and limited treatment accessibility remain significant restraints in the global Hyaluronidase Deficiency market. Developing countries, in particular, face challenges in affording advanced biologic therapies due to limited healthcare funding, inadequate insurance coverage, and high import costs associated with specialized enzyme formulations
- For instance, in many low- and middle-income regions, patients with rare metabolic disorders often experience delayed diagnosis and limited access to recombinant enzyme therapies, which are typically available only in specialized treatment centers. This disparity contributes to unmet medical needs and restricts the overall market growth potential
- Another major challenge lies in the complex manufacturing process of recombinant enzymes, which involves high production costs, stringent regulatory compliance, and extended clinical validation timelines. These factors collectively lead to elevated market entry barriers for new players
- Furthermore, limited awareness among healthcare providers and patients regarding enzyme deficiency symptoms and treatment options results in underdiagnosis and undertreatment. The lack of standardized treatment guidelines and insufficient reimbursement frameworks also hinders therapy adoption
- Addressing these challenges requires a multi-dimensional approach involving price optimization, healthcare education, and policy support for rare disease management. Collaborative efforts between governments, biopharma companies, and international health organizations to subsidize treatment costs and enhance diagnostic capabilities will be crucial for improving patient access and achieving sustained market growth
Hyaluronidase Deficiency Market Scope
The market is segmented on the basis of symptoms, type, and application.
- By Symptoms
On the basis of symptoms, the Hyaluronidase Deficiency market is segmented into Short Stature, Mildly Dysmorphic Facial Features, Soft Tissue Masses, and Knee and Hip Pain. The Short Stature segment dominated the largest market revenue share of 38.6% in 2024, primarily due to the high prevalence of growth-related abnormalities among pediatric patients diagnosed with enzymatic disorders. Early clinical screening and genetic testing advancements have enhanced the identification of individuals with growth retardation caused by hyaluronidase deficiency. The rise in awareness about developmental anomalies, along with improved access to metabolic diagnostic centers, has also supported this segment’s market strength. Furthermore, ongoing clinical trials focusing on enzyme replacement therapy (ERT) for genetic short stature disorders are increasing therapeutic adoption rates. Healthcare professionals increasingly associate short stature with hyaluronidase gene mutations, driving higher diagnosis rates. In addition, expanding research collaborations between genetic laboratories and academic institutions are accelerating early-stage detection, further supporting dominance. Increased healthcare expenditure and public health initiatives in developed economies have ensured better treatment availability, solidifying this segment’s leadership in 2024.
The Soft Tissue Masses segment is anticipated to witness the fastest growth rate of 9.2% CAGR from 2025 to 2032, owing to growing recognition of abnormal tissue accumulation and swelling as key manifestations of enzyme deficiency. Soft tissue masses, often linked to impaired extracellular matrix degradation, have become a focal point of medical attention due to their diagnostic complexity. Rising clinical awareness and utilization of imaging tools such as MRI and ultrasound are aiding in early detection. Increased focus on differential diagnosis and the use of histopathological examination in suspected cases are fueling accurate case identification. Moreover, advancements in recombinant enzyme-based treatments have created new therapeutic opportunities. Pharmaceutical and biotech companies are investing in studies targeting extracellular tissue buildup, leading to better management outcomes. Patients increasingly seek specialized care as these masses affect joint flexibility and mobility, further boosting demand. The development of synthetic enzymes and targeted delivery systems is projected to enhance treatment precision, driving substantial segment growth through 2032.
- By Type
On the basis of type, the Hyaluronidase Deficiency market is segmented into Animal-Derived Hyaluronidase and Synthetic Hyaluronidase. The Animal-Derived Hyaluronidase segment dominated the market with the largest revenue share of 56.4% in 2024, owing to its established clinical usage, cost efficiency, and broad therapeutic availability. Derived primarily from bovine and ovine sources, these enzymes have been utilized for decades in medical and cosmetic applications, ensuring physician trust and familiarity. Regulatory approvals for animal-based formulations across multiple countries continue to support their clinical preference. In addition, the affordability of manufacturing and the extensive historical data on safety and efficacy make these formulations a preferred choice in both hospital and outpatient settings. Developing economies still rely heavily on animal-derived enzymes due to limited access to advanced synthetic options. Moreover, established supply chains and a stable global distribution network further strengthen this segment’s dominance. Increased demand from dermatology and ophthalmology sectors for enzyme-based adjuvants has also boosted consumption levels. Despite emerging alternatives, the reliability and accessibility of animal-derived formulations maintain their market leadership globally.
The Synthetic Hyaluronidase segment is projected to record the fastest CAGR of 10.8% from 2025 to 2032, driven by technological advancements in recombinant DNA technology and enzyme bioengineering. Synthetic enzymes are increasingly favored due to their enhanced purity, controlled activity, and lower immunogenic potential compared to animal-derived variants. Growing preference for biocompatible and non-animal-based formulations in developed regions supports strong adoption rates. Pharmaceutical manufacturers are investing heavily in developing synthetic hyaluronidase for use in oncology, ophthalmology, and aesthetics. Furthermore, increasing regulatory approvals for recombinant enzyme formulations across the U.S. and Europe have accelerated their commercialization. Clinical studies demonstrate superior stability and safety of synthetic enzymes, leading to higher physician confidence. Strategic partnerships between biotech companies and research institutions are expediting product innovation. The rising global shift toward ethical and sustainable biomanufacturing further enhances the synthetic segment’s attractiveness. By 2032, this category is anticipated to replace animal-derived enzymes in several therapeutic fields, highlighting its strong growth potential.
- By Application
On the basis of application, the Hyaluronidase Deficiency market is segmented into Dermatology, Chemotherapy, Ophthalmology, Plastic Surgery, and Others. The Dermatology segment accounted for the largest market revenue share of 41.3% in 2024, driven by the rising prevalence of connective tissue disorders and skin-related complications caused by enzyme insufficiency. Hyaluronidase plays a critical role in maintaining extracellular matrix balance, and its deficiency often results in skin thickening, swelling, or fibrosis, prompting dermatological interventions. Increasing awareness among dermatologists about enzyme-related dermal abnormalities has improved patient management outcomes. Moreover, enzyme therapy is widely used in aesthetic medicine to address dermal filler complications and improve tissue permeability. The continuous expansion of dermatology clinics, combined with the growing patient pool seeking corrective and therapeutic procedures, further supports segment leadership. Advances in topical enzyme formulations and transdermal delivery technologies are enhancing treatment efficacy and patient compliance. In addition, robust clinical research exploring combination therapies involving hyaluronidase has strengthened its medical relevance. With growing public awareness about skin health, this segment is expected to maintain its dominance through sustained demand for enzyme-based skincare therapies.
The Ophthalmology segment is anticipated to register the fastest CAGR of 9.7% from 2025 to 2032, owing to the increasing use of hyaluronidase in ocular surgeries and drug delivery. The enzyme facilitates better absorption of ophthalmic drugs by breaking down extracellular matrix barriers, improving permeability. Its use in enhancing local anesthetic diffusion and reducing post-surgical edema has made it essential in ophthalmic care. Growing incidences of eye disorders associated with connective tissue dysfunction have amplified therapeutic needs. Researchers are actively investigating the potential of synthetic and recombinant hyaluronidase in intravitreal injections to improve treatment outcomes for retinal diseases. Furthermore, expanding ophthalmology research in Asia-Pacific and Europe is driving innovation in enzyme-based eye care solutions. Continuous advancements in enzyme delivery systems, including microinjection and nanoformulations, enhance precision and therapeutic safety. Increased training among ophthalmic specialists and favorable reimbursement policies are also supporting this segment’s expansion. As ophthalmic drug administration becomes more enzyme-assisted, this category is projected to exhibit consistent and high growth through 2032.
Hyaluronidase Deficiency Market Regional Analysis
- North America dominated the hyaluronidase deficiency market with the largest revenue share of 41.6% in 2024, supported by strong research infrastructure, a well-established biopharmaceutical ecosystem, and increasing awareness regarding genetic enzyme deficiencies. The region’s leadership is primarily attributed to high investment in enzyme replacement therapy (ERT) development and advanced diagnostic technologies that facilitate early identification of enzyme-related disorders
- Government support for orphan drug development and favorable regulatory pathways by agencies such as the U.S. FDA continue to encourage innovation and accelerate therapy approvals
- The growing presence of major biotechnology companies—such as Halozyme Therapeutics, Baxter International, and AbbVie—with active research portfolios in recombinant enzyme and biologics development further enhances regional dominance
U.S. Hyaluronidase Deficiency Market Insight
The U.S. hyaluronidase deficiency market captured the largest share of over 80% within North America in 2024, driven by a robust clinical research pipeline, active funding for rare disease programs, and favorable government initiatives such as the Orphan Drug Act. The country’s expanding network of enzyme therapy providers and diagnostic laboratories enables early intervention and specialized patient care. The U.S. also leads in genetic sequencing advancements, which are improving diagnosis accuracy and enabling personalized enzyme therapy approaches. Partnerships between biotech startups and large pharmaceutical firms continue to accelerate innovation, ensuring sustained market leadership in the forecast period.
Europe Hyaluronidase Deficiency Market Insight
The Europe hyaluronidase deficiency market expected to grow steadily during the forecast period, supported by strong public health initiatives, enhanced access to rare disease treatment programs, and increased clinical awareness. Countries such as Germany, the U.K., and France are leading contributors, benefiting from progressive healthcare systems and well-structured reimbursement models for enzyme replacement therapies. The growing adoption of recombinant enzyme therapies and increased participation in global clinical trials are further propelling market growth. Europe’s regulatory environment, led by the European Medicines Agency (EMA), continues to support innovation through orphan drug designations and incentives for biopharmaceutical developers. Rising patient advocacy, genetic testing programs, and expanding clinical infrastructure across Western and Northern Europe are expected to drive consistent growth through 2032.
U.K. Hyaluronidase Deficiency Market Insight
The U.K. hyaluronidase deficiency market is anticipated to grow at a significant CAGR during 2025–2032, driven by the National Health Service’s (NHS) increasing support for rare disease management and improved accessibility to biologic therapies. The U.K.’s emphasis on early screening, along with investments in genomics research such as the Genomics England Initiative, is enhancing diagnostic accuracy and patient outcomes. The rise in partnerships between biotechnology firms and academic research centers is expected to further strengthen innovation in enzyme replacement and gene therapy development.
Germany Hyaluronidase Deficiency Market Insight
The Germany hyaluronidase deficiency market is projected to expand steadily, backed by the nation’s strong biopharmaceutical manufacturing base, advanced research infrastructure, and emphasis on innovation in enzyme engineering. Germany’s focus on sustainable bioprocessing and recombinant protein technologies supports large-scale production efficiency. The country’s healthcare system ensures comprehensive coverage for rare metabolic disorders, increasing access to treatment. Collaborative efforts between leading research universities and global pharma players such as Bayer AG and BioNTech SE are expected to further boost growth.
Asia-Pacific Hyaluronidase Deficiency Market Insight
The Asia-Pacific hyaluronidase deficiency market is projected to register the fastest CAGR of 8.9% from 2025 to 2032, driven by improving healthcare infrastructure, growing investment in rare disease research, and increased early diagnosis initiatives in emerging economies. The region’s expanding biotechnology industry, coupled with rising government support for orphan drug development, is creating a strong foundation for growth. Countries such as Japan, China, and South Korea are at the forefront, leveraging advancements in genetic sequencing and recombinant technology to develop cost-effective therapies. The increasing participation of Asian firms in global clinical trials and the establishment of regional manufacturing hubs are improving affordability and access to enzyme therapies. Furthermore, awareness campaigns and patient advocacy programs are helping overcome diagnostic delays and encouraging timely treatment adoption. The region’s focus on healthcare modernization and localized biomanufacturing will continue to accelerate market expansion through 2032.
Japan Hyaluronidase Deficiency Market Insight
The Japan hyaluronidase deficiency market is witnessing robust growth due to the country’s advanced healthcare infrastructure, strong emphasis on biotechnology innovation, and rapid adoption of genetic testing. Japan’s regulatory reforms to support rare disease treatments and reimbursement programs for high-cost enzyme therapies are boosting access. In addition, domestic pharmaceutical companies are collaborating with international biotechs to co-develop enzyme-based and gene therapies, positioning Japan as a major contributor to the regional market’s expansion.
China Hyaluronidase Deficiency Market Insight
The China hyaluronidase deficiency market accounted for the largest revenue share in Asia-Pacific in 2024, supported by rapid urbanization, growing healthcare investments, and an expanding middle-class population seeking access to advanced biologic treatments. Government policies promoting innovation, such as the Made in China 2025 initiative, have led to the development of local enzyme therapy manufacturing capabilities. Increased partnerships between Chinese biotech companies and global pharmaceutical leaders are fostering technology transfer and cost-efficient production, enhancing treatment accessibility and supporting market growth throughout the forecast period.
Hyaluronidase Deficiency Market Share
The Hyaluronidase Deficiency industry is primarily led by well-established companies, including:
- Halozyme Therapeutics, Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Bausch Health Companies Inc. (Canada)
- Roche Holding AG (Switzerland)
- Pfizer Inc. (U.S.)
- Baxter International Inc. (U.S.)
- Amphastar Pharmaceuticals, Inc. (U.S.)
- STEMCELL Technologies Inc. (Canada)
- AbbVie Inc. (U.S.)
- Merck KGaA (Germany)
- Cipla Limited (India)
- Johnson & Johnson Services, Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sandoz International GmbH (Germany)
- Ferring Pharmaceuticals (Switzerland)
- Catalent, Inc. (U.S.)
- Arysta LifeScience (Japan)
- Dr. Reddy’s Laboratories Ltd. (India)
- Novartis AG (Switzerland)
Latest Developments in Global Hyaluronidase Deficiency Market
- In October 2023, Bristol-Myers Squibb announced that the Phase 3 CheckMate-67T trial of subcutaneous nivolumab co-formulated with recombinant human hyaluronidase (rHuPH20) met its co-primary pharmacokinetics endpoints in patients with advanced or metastatic clear-cell renal cell carcinoma. This milestone highlighted the growing role of hyaluronidase in enhancing biologic drug delivery efficiency and patient convenience
- In May 2024, the U.S. Food and Drug Administration (FDA) announced an updated PDUFA goal date of December 29, 2024 for the biologics license application (BLA) of subcutaneous nivolumab (nivolumab + rHuPH20). This update emphasized the ongoing regulatory momentum for hyaluronidase-enabled subcutaneous biologic therapies
- In January 2024, the FDA approved IgG-hyaluronidase recombinant, a formulation combining immunoglobulin and recombinant human hyaluronidase, for the maintenance treatment of adult patients with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP). The approval expanded the clinical scope of recombinant hyaluronidase technology into neuromuscular disorders
- In September 2024, Roche announced FDA approval of OCREVUS ZUNOVO (ocrelizumab & hyaluronidase-ocsq), a subcutaneous multiple sclerosis therapy that leverages hyaluronidase enzyme technology (rHuPH20) to enable faster, less invasive dosing compared to traditional intravenous administration
- In May 2025, a pre-clinical study reported the successful development of a rapid, high-volume auto-injector system capable of administering large-volume biologic formulations co-delivered with recombinant human hyaluronidase (rHuPH20). The findings demonstrated the potential for improved patient comfort and delivery efficiency in enzyme-facilitated subcutaneous drug administration
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.