Global Interventional Cardiology Market
Market Size in USD Billion
CAGR :
% 
 USD
14.61 Billion
USD
23.61 Billion
2022
2030
USD
14.61 Billion
USD
23.61 Billion
2022
2030
| 2023 –2030 | |
| USD 14.61 Billion | |
| USD 23.61 Billion | |
|
|
|
|
Interventional Cardiology Market Analysis and Size
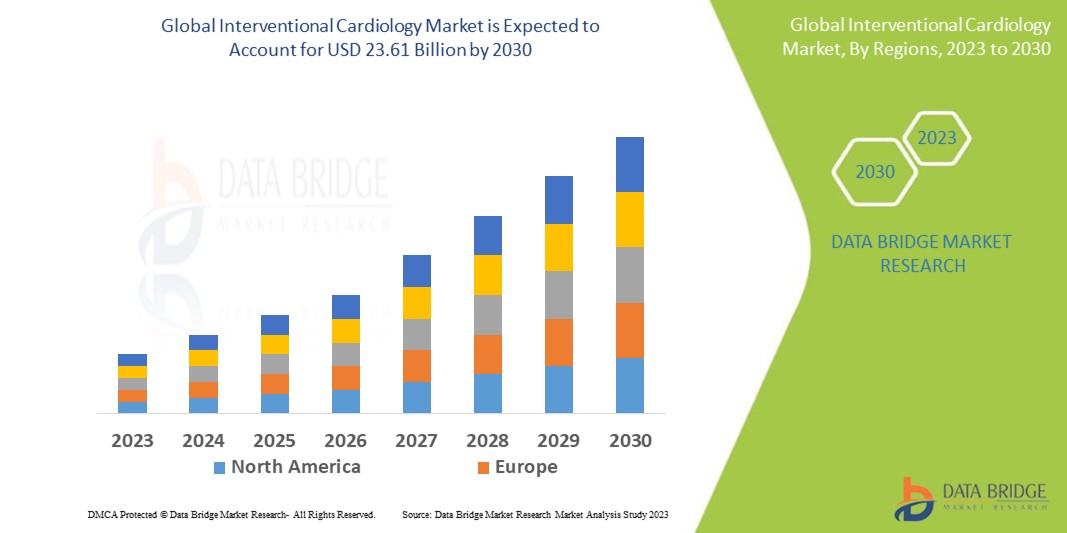
The increasing prevalence of cardiovascular diseases and technological advancements have significantly improved the design and functionality of the interventional cardiology market and have carved the way for market growth. In addition, the rising healthcare expenditure and the increasing geriatric population contribute to the market growth.
Data Bridge Market Research analyzes that the global interventional cardiology market, which was USD 14.61 billion in 2022, would rocket up to USD 23.61 billion by 2030, and is expected to undergo a CAGR of 7.1% during the forecast period. This indicates that the conventional device segment dominates the device type segment of the market owing to the advancements in technology that have led to the development of advancements in interventional cardiology for various use.
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Interventional Cardiology Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2030) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
By Device Type (Conventional Device, Advanced Device), End User (Hospitals, Clinics, Cardiac Catheterization Laboratories, Ambulatory Surgical Centres, Others) |
|
Countries Covered |
U.S., Canada, Mexico, Brazil, Argentina, Peru, Rest of South America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia-Pacific, Saudi Arabia, U.A.E., Egypt, Israel, Kuwait, South Africa, and Rest of Middle East and Africa |
|
Market Players Covered |
ABIOMED, Inc. (U.S.), ACIST Medical Systems (U.S.), B. Braun Melsungen AG (U.S.), Biotronik SE & Co. KG (Germany), Boston Scientific Corporation (U.S.), BD (U.S.), Cook (U.S.), Cardinal Health (U.S.), Edwards Lifesciences Corporation (U.S.), Ivascular (Spain), S.L.U. (U.S.), Johnson & Johnson Services, Inc. (U.S.), Medtronic (U.S.), Merit Medical Systems (U.S.), Koninklijke Philips N.V. (U.S.), Abbott (U.S.), Terumo Corporation (Japan), Rontis Corporation (Switzerland), W. L. Gore & Associates, Inc. (U.S.), Biosensors International Group, Ltd. (U.S.), and GENERAL ELECTRIC COMPANY (U.S.) among others |
|
Market Opportunities |
|
Market Definition
Interventional cardiology is a branch of cardiology that focuses on diagnosing and treating cardiovascular diseases using minimally invasive techniques. The global interventional cardiology market refers to the worldwide market for medical devices and procedures used in interventional cardiology. Physicians use catheters (thin, flexible tubes) and other medical devices in interventional cardiology to access and treat the heart and blood vessels. These procedures are typically performed through small incisions or punctures in the skin rather than through open surgery, which helps reduce risks, complications, and recovery time for patients.
Global Interventional Cardiology Market Dynamics
Drivers
- Increasing prevalence of cardiovascular diseases
The rising incidence of cardiovascular diseases, such as coronary artery disease, heart attacks, and cardiac arrhythmias, is a major driver for market growth. The need for effective diagnosis and treatment of these conditions has led to the growth in demand for interventional cardiology procedures.
- Preference for minimally invasive procedures
Interventional cardiology procedures are minimally invasive, meaning they involve smaller incisions and have shorter recovery times compared to traditional open-heart surgeries. As patients and physicians increasingly prefer minimally invasive procedures, the demand for interventional cardiology is expected to rise.
- Technological advancements
Advances in interventional cardiology technologies and techniques have greatly improved the outcomes of cardiac procedures. Innovations such as drug-eluting stents, bioresorbable stents, and Transcatheter Aortic Valve Replacement (TAVR) have revolutionized the field, attracting both patients and healthcare providers to interventional cardiology procedures.
Opportunities
- Increasing healthcare expenditure
Increased healthcare spending, particularly in developing countries, has led to improved access to advanced cardiac care, including interventional cardiology. As governments and healthcare organizations invest in upgrading medical infrastructure and facilities, the interventional cardiology market is likely to expand.
- Growing elderly population
The global population is aging, and elderly individuals are more susceptible to cardiovascular diseases. With the increasing aging population, there is a higher demand for interventional cardiology procedures to diagnose and treat cardiac conditions in this group, driving the market growth.
Restraint
- High cost of interventional procedures
Interventional cardiology procedures, such as angioplasty and stenting, can be costly. The high cost can limit access to these procedures in developing countries and create financial burdens for patients and healthcare systems in general.
Challenge
- Stringent regulatory approval processes
The development and commercialization of interventional cardiology devices require extensive clinical trials and regulatory approvals. Stringent regulatory processes can significantly delay the introduction of new devices and technologies to the market, affecting the growth potential of the industry.
This global interventional cardiology market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on this market, contact Data Bridge Market Research for an Analyst Brief. Our team will help you make an informed market decision to achieve market growth.
Recent Developments
- In May 2023, Abbott announced a series of new programs within its multi-million-dollar initiative to increase diversity in clinical trials and improve care among under-represented populations
- In May 2022, Babson Diagnostics, a science-first healthcare technology company, and BD, a leading global medical technology company, announced the expansion of a strategic partnership to move blood sample collection into new care settings, including enabling patients to collect blood samples at home for diagnostic testing.
Global Interventional Cardiology Market Scope
The global interventional cardiology market is segmented into two notable segments based on device type and end user. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Device Type
- Conventional Device
- Advanced Device
End User
- Hospitals
- Clinics
- Cardiac Catheterization Laboratories
- Ambulatory Surgical Centres
- Others
Global Interventional Cardiology Market Regional Analysis/Insights
The global interventional cardiology market is analysed and market size insights and trends are provided by device type and end user as referenced above.
The countries covered in this market report are U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, U.A.E., Egypt, Israel, and Rest of Middle East and Africa.
North America dominates the global interventional cardiology market due to the presence of major key players, high disposable income, high healthcare expenditure, and a well-developed healthcare sector in this region.
Asia-Pacific is expected to grow during the forecast period of 2023-2030 due to increasing R&D activities, rising investment in the healthcare sector, and growing government support.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The global interventional cardiology market also provides you with a detailed market analysis for every country's growth in healthcare expenditure for capital equipment, installed base of different kinds of products for the market, the impact of technology using lifeline curves, and changes in healthcare regulatory scenarios and their impact on the market. The data is available for the historic period 2015-2020.
Competitive Landscape and Global Interventional Cardiology Market Share Analysis
The global interventional cardiology market competitive landscape provides details by competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus related to market .
Some of the major players operating in the global interventional cardiology market are:
- ABIOMED, Inc. (U.S.)
- ACIST Medical Systems (U.S.)
- B. Braun Melsungen AG (U.S.)
- Biotronik SE & Co. KG (Germany)
- Boston Scientific Corporation (U.S.)
- BD (U.S.)
- Cook (U.S.)
- Cardinal Health (U.S.)
- Edwards Lifesciences Corporation (U.S.)
- Ivascular (Spain)
- Johnson & Johnson Services, Inc. (U.S.)
- Medtronic (U.S.)
- Merit Medical Systems (U.S.)
- Koninklijke Philips N.V. (U.S.)
- Abbott (U.S.)
- Terumo Corporation (Japan)
- Rontis Corporation (Switzerland)
- W. L. Gore & Associates, Inc. (U.S.)
- Biosensors International Group, Ltd. (U.S.)
- GENERAL ELECTRIC COMPANY (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1. INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL INTERVENTIONAL CARDIOLOGY MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2. MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL INTERVENTIONAL CARDIOLOGY MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES DATA VOLUME
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL INTERVENTIONAL CARDIOLOGY MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3. MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4. EXECUTIVE SUMMARY
5. PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S 5 FORCES MODEL
5.3 STRATEGIC INITIATIVES
6. REGULATORY FRAMWORK
7. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, BY PRODUCT
7.1 OVERVIEW
(NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS OF PRODUCT)
7.2 GUIDE WIRES
7.2.1 CORONARY GUIDE WIRES
7.2.1.1. MARKET VALUE (USD MILLION)
7.2.1.2. MARKET VALUE (UNITS)
7.2.1.3. AVERAGE SELLING PRICE (USD)
7.2.2 SPECIALTY GUIDE WIRES
7.2.2.1. MARKET VALUE (USD MILLION)
7.2.2.2. MARKET VALUE (UNITS)
7.2.2.3. AVERAGE SELLING PRICE (USD)
7.3 CATHETERS
7.3.1 BALLOON DILATATION CATHETERS
7.3.1.1. MARKET VALUE (USD MILLION)
7.3.1.2. MARKET VALUE (UNITS)
7.3.1.3. AVERAGE SELLING PRICE (USD)
7.3.2 ANGIOGRAPHY CATHETERS
7.3.2.1. MARKET VALUE (USD MILLION)
7.3.2.2. MARKET VALUE (UNITS)
7.3.2.3. AVERAGE SELLING PRICE (USD)
7.3.3 INTRAVASCULAR ULTRASOUND (IVUS) CATHETERS
7.3.3.1. MARKET VALUE (USD MILLION)
7.3.3.2. MARKET VALUE (UNITS)
7.3.3.3. AVERAGE SELLING PRICE (USD)
7.3.4 PERCUTANEOUS TRANSLUMINAL CORONARY ANGIOPLASTY (PTCA) GUIDING CATHETERS
7.3.4.1. MARKET VALUE (USD MILLION)
7.3.4.2. MARKET VALUE (UNITS)
7.3.4.3. AVERAGE SELLING PRICE (USD)
7.3.5 GUIDE EXTENSION CATHETERS
7.3.5.1. MARKET VALUE (USD MILLION)
7.3.5.2. MARKET VALUE (UNITS)
7.3.5.3. AVERAGE SELLING PRICE (USD)
7.3.6 MICROCATHETERS
7.3.6.1. MARKET VALUE (USD MILLION)
7.3.6.2. MARKET VALUE (UNITS)
7.3.6.3. AVERAGE SELLING PRICE (USD)
7.3.7 DUAL LUMEN CATHETERS
7.3.7.1. MARKET VALUE (USD MILLION)
7.3.7.2. MARKET VALUE (UNITS)
7.3.7.3. AVERAGE SELLING PRICE (USD)
7.3.8 OTHERS
7.4 CORONARY STENTS
7.4.1 BY TYPE
7.4.1.1. BARE STENTS
7.4.1.1.1. MARKET VALUE (USD MILLION)
7.4.1.1.2. MARKET VALUE (UNITS)
7.4.1.1.3. AVERAGE SELLING PRICE (USD)
7.4.1.2. DRUG-ELUTING STENTS
7.4.1.2.1. BIODEGRADABLE
7.4.1.2.1.1 MARKET VALUE (USD MILLION)
7.4.1.2.1.2 MARKET VALUE (UNITS)
7.4.1.2.1.3 AVERAGE SELLING PRICE (USD)
7.4.1.2.2. NON-BIODEGRADABLE
7.4.1.2.2.1 MARKET VALUE (USD MILLION)
7.4.1.2.2.2 MARKET VALUE (UNITS)
7.4.1.2.2.3 AVERAGE SELLING PRICE (USD)
7.4.1.3. BIOENGINEERED STENTS
7.4.1.3.1. MARKET VALUE (USD MILLION)
7.4.1.3.2. MARKET VALUE (UNITS)
7.4.1.3.3. AVERAGE SELLING PRICE (USD)
7.4.1.4. DUAL THERAPY STENTS
7.4.1.4.1. MARKET VALUE (USD MILLION)
7.4.1.4.2. MARKET VALUE (UNITS)
7.4.1.4.3. AVERAGE SELLING PRICE (USD)
7.4.2 BY MATERIAL
7.4.2.1. METALLIC MATERIALS
7.4.2.1.1. STAINLESS STEEL
7.4.2.1.1.1 MARKET VALUE (USD MILLION)
7.4.2.1.1.2 MARKET VALUE (UNITS)
7.4.2.1.1.3 AVERAGE SELLING PRICE (USD)
7.4.2.1.2. COBALT ALLOY METAL
7.4.2.1.2.1 MARKET VALUE (USD MILLION)
7.4.2.1.2.2 MARKET VALUE (UNITS)
7.4.2.1.2.3 AVERAGE SELLING PRICE (USD)
7.4.2.1.3. GOLD
7.4.2.1.3.1 MARKET VALUE (USD MILLION)
7.4.2.1.3.2 MARKET VALUE (UNITS)
7.4.2.1.3.3 AVERAGE SELLING PRICE (USD)
7.4.2.1.4. TANTALUM
7.4.2.1.4.1 MARKET VALUE (USD MILLION)
7.4.2.1.4.2 MARKET VALUE (UNITS)
7.4.2.1.4.3 AVERAGE SELLING PRICE (USD)
7.4.2.1.5. PLATINUM CHROMIUM
7.4.2.1.5.1 MARKET VALUE (USD MILLION)
7.4.2.1.5.2 MARKET VALUE (UNITS)
7.4.2.1.5.3 AVERAGE SELLING PRICE (USD)
7.4.2.1.6. NICKEL TITANIUM
7.4.2.1.6.1 MARKET VALUE (USD MILLION)
7.4.2.1.6.2 MARKET VALUE (UNITS)
7.4.2.1.6.3 AVERAGE SELLING PRICE (USD)
7.4.2.2. POLYMERS BIOMATERIALS
7.4.2.2.1. MARKET VALUE (USD MILLION)
7.4.2.2.2. MARKET VALUE (UNITS)
7.4.2.2.3. AVERAGE SELLING PRICE (USD)
7.4.2.3. NATURAL BIOMATERIALS
7.4.2.3.1. MARKET VALUE (USD MILLION)
7.4.2.3.2. MARKET VALUE (UNITS)
7.4.2.3.3. AVERAGE SELLING PRICE (USD)
7.4.3 BY MODE OF DELIVERY
7.4.3.1. BALLOON EXPANDABLE STENTS
7.4.3.1.1. MARKET VALUE (USD MILLION)
7.4.3.1.2. MARKET VALUE (UNITS)
7.4.3.1.3. AVERAGE SELLING PRICE (USD)
7.4.3.2. SELF EXPANDING STENTS
7.4.3.2.1. MARKET VALUE (USD MILLION)
7.4.3.2.2. MARKET VALUE (UNITS)
7.4.3.2.3. AVERAGE SELLING PRICE (USD)
7.5 VASCULAR CLOSURE DEVICES (VCDS)
7.5.1 BY TYPE
7.5.1.1. COLLAGEN PLUG-BASED (ANGIO-SEAL)
7.5.1.1.1. MARKET VALUE (USD MILLION)
7.5.1.1.2. MARKET VALUE (UNITS)
7.5.1.1.3. AVERAGE SELLING PRICE (USD)
7.5.1.2. SEALANT OR GEL BASED DEVICES
7.5.1.2.1. MARKET VALUE (USD MILLION)
7.5.1.2.2. MARKET VALUE (UNITS)
7.5.1.2.3. AVERAGE SELLING PRICE (USD)
7.5.1.3. COMPRESSION ASSISTED DEVICES
7.5.1.3.1. MARKET VALUE (USD MILLION)
7.5.1.3.2. MARKET VALUE (UNITS)
7.5.1.3.3. AVERAGE SELLING PRICE (USD)
7.5.1.4. SUTURE BASED (PERCLOSE)
7.5.1.4.1. MARKET VALUE (USD MILLION)
7.5.1.4.2. MARKET VALUE (UNITS)
7.5.1.4.3. AVERAGE SELLING PRICE (USD)
7.5.1.5. NITINOL CLIP-BASED (STARCLOSE) DEVICES
7.5.1.5.1. MARKET VALUE (USD MILLION)
7.5.1.5.2. MARKET VALUE (UNITS)
7.5.1.5.3. AVERAGE SELLING PRICE (USD)
7.5.2 BY MODE OF ACTION
7.5.2.1. FEMORAL ACCESS
7.5.2.1.1. MARKET VALUE (USD MILLION)
7.5.2.1.2. MARKET VALUE (UNITS)
7.5.2.1.3. AVERAGE SELLING PRICE (USD)
7.5.2.2. RADIAL ACCESS
7.5.2.2.1. MARKET VALUE (USD MILLION)
7.5.2.2.2. MARKET VALUE (UNITS)
7.5.2.2.3. AVERAGE SELLING PRICE (USD)
7.6 INTRAVASCULAR ULTRASOUND (IVUS)
7.6.1 MARKET VALUE (USD MILLION)
7.6.2 MARKET VALUE (UNITS)
7.6.3 AVERAGE SELLING PRICE (USD)
7.7 FRACTIONAL FLOW RESERVE (FFR)
7.7.1 MARKET VALUE (USD MILLION)
7.7.2 MARKET VALUE (UNITS)
7.7.3 AVERAGE SELLING PRICE (USD)
7.8 OPTICAL COHERENCE TOMOGRAPHY (OCT)
7.8.1 MARKET VALUE (USD MILLION)
7.8.2 MARKET VALUE (UNITS)
7.8.3 AVERAGE SELLING PRICE (USD)
7.9 ACCESSORIES
7.9.1 BALLOON INFLATION DEVICES
7.9.1.1. MARKET VALUE (USD MILLION)
7.9.1.2. MARKET VALUE (UNITS)
7.9.1.3. AVERAGE SELLING PRICE (USD)
7.9.2 ANGIOPLASTY KITS
7.9.2.1. MARKET VALUE (USD MILLION)
7.9.2.2. MARKET VALUE (UNITS)
7.9.2.3. AVERAGE SELLING PRICE (USD)
7.9.3 INTRODUCER SHEATHS
7.9.3.1. MARKET VALUE (USD MILLION)
7.9.3.2. MARKET VALUE (UNITS)
7.9.3.3. AVERAGE SELLING PRICE (USD)
7.9.4 OTHERS
8. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, BY TYPE
8.1 OVERVIEW
8.2 CONVENTIONAL
8.2.1 CORONARY GUIDE WIRES
8.2.2 CATHETERS
8.2.3 CORONARY STENTS
8.2.4 VASCULAR CLOSURE DEVICES (VCDS)
8.2.5 OTHERS
8.3 ADVANCED
8.3.1 INTRAVASCULAR ULTRASOUND (IVUS)
8.3.2 FRACTIONAL FLOW RESERVE (FFR)
8.3.3 OPTICAL COHERENCE TOMOGRAPHY (OCT)
9. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, BY INDICATION
9.1 OVERVIEW
9.2 ISCHEMIC HEART DISEASE
9.3 PERCUTANEOUS CORONARY INTERVENTION
9.4 STENT PLACEMENT
9.5 CORONARY THROMBECTOMY
9.6 CONGENITAL HEART ABNORMALITIES
9.7 OTHERS
10. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, BY AGE
10.1 OVERVIEW
10.2 ADULT
10.3 PEDIATRIC
10.4 GERIARTIC
11. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.2.1 ACUTE CARE HOSPITALS
11.2.2 LONG-TERM CARE HOSPITALS
11.3 CATH LABORATORIES
11.4 AMBULATORY SURGICAL CENTERS
11.5 ACADEMIC & RESEARCH INSTITUTES
11.6 OTHERS
12. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDERS
12.3 RETAIL SALES
12.4 OTHERS
13. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, BY COUNTRY
GLOBAL INTERVENTIONAL CARDIOLOGY MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
13.1 NORTH AMERICA
13.1.1 U.S.
13.1.2 CANADA
13.1.3 MEXICO
13.2 EUROPE
13.2.1 GERMANY
13.2.2 U.K.
13.2.3 ITALY
13.2.4 FRANCE
13.2.5 SPAIN
13.2.6 RUSSIA
13.2.7 SWITZERLAND
13.2.8 TURKEY
13.2.9 BELGIUM
13.2.10 NETHERLANDS
13.2.11 DENMARK
13.2.12 SWEDEN
13.2.13 POLAND
13.2.14 NORWAY
13.2.15 FINLAND
13.2.16 REST OF EUROPE
13.3 ASIA-PACIFIC
13.3.1 JAPAN
13.3.2 CHINA
13.3.3 SOUTH KOREA
13.3.4 INDIA
13.3.5 SINGAPORE
13.3.6 THAILAND
13.3.7 INDONESIA
13.3.8 MALAYSIA
13.3.9 PHILIPPINES
13.3.10 AUSTRALIA
13.3.11 NEW ZEALAND
13.3.12 VIETNAM
13.3.13 TAIWAN
13.3.14 REST OF ASIA-PACIFIC
13.4 SOUTH AMERICA
13.4.1 BRAZIL
13.4.2 ARGENTINA
13.4.3 REST OF SOUTH AMERICA
13.5 MIDDLE EAST AND AFRICA
13.5.1 SOUTH AFRICA
13.5.2 EGYPT
13.5.3 BAHRAIN
13.5.4 UNITED ARAB EMIRATES
13.5.5 KUWAIT
13.5.6 OMAN
13.5.7 QATAR
13.5.8 SAUDI ARABIA
13.5.9 REST OF MEA
13.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
14. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, SWOT AND DBMR ANALYSIS
15. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: GLOBAL
15.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
15.3 COMPANY SHARE ANALYSIS: EUROPE
15.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15.5 MERGERS & ACQUISITIONS
15.6 NEW PRODUCT DEVELOPMENT & APPROVALS
15.7 EXPANSIONS
15.8 REGULATORY CHANGES
15.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
16. GLOBAL INTERVENTIONAL CARDIOLOGY MARKET, COMPANY PROFILE
16.1 ABBOTT LABORATORIES
16.1.1 COMPANY OVERVIEW
16.1.2 REVENUE ANALYSIS
16.1.3 GEOGRAPHIC PRESENCE
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPEMENTS
16.2 B. BRAUN MELSUNGEN AG
16.2.1 COMPANY OVERVIEW
16.2.2 REVENUE ANALYSIS
16.2.3 GEOGRAPHIC PRESENCE
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPEMENTS
16.3 BIOSENSORS INTERNATIONAL GROUP LTD
16.3.1 COMPANY OVERVIEW
16.3.2 REVENUE ANALYSIS
16.3.3 GEOGRAPHIC PRESENCE
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPEMENTS
16.4 BIOTRONIK
16.4.1 COMPANY OVERVIEW
16.4.2 REVENUE ANALYSIS
16.4.3 GEOGRAPHIC PRESENCE
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPEMENTS
16.5 BD
16.5.1 COMPANY OVERVIEW
16.5.2 REVENUE ANALYSIS
16.5.3 GEOGRAPHIC PRESENCE
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPEMENTS
16.6 BOSTON SCIENTIFIC
16.6.1 COMPANY OVERVIEW
16.6.2 REVENUE ANALYSIS
16.6.3 GEOGRAPHIC PRESENCE
16.6.4 PRODUCT PORTFOLIO
16.6.5 RECENT DEVELOPEMENTS
16.7 CARDINAL HEALTH
16.7.1 COMPANY OVERVIEW
16.7.2 REVENUE ANALYSIS
16.7.3 GEOGRAPHIC PRESENCE
16.7.4 PRODUCT PORTFOLIO
16.7.5 RECENT DEVELOPEMENTS
16.8 COOK MEDICAL INC.
16.8.1 COMPANY OVERVIEW
16.8.2 REVENUE ANALYSIS
16.8.3 GEOGRAPHIC PRESENCE
16.8.4 PRODUCT PORTFOLIO
16.8.5 RECENT DEVELOPEMENTS
16.9 MICROPORT SCIENTIFIC CORP.
16.9.1 T COMPANY OVERVIEW
16.9.2 REVENUE ANALYSIS
16.9.3 GEOGRAPHIC PRESENCE
16.9.4 PRODUCT PORTFOLIO
16.9.5 RECENT DEVELOPEMENTS
16.10 TELEFLEX INC.
16.10.1 COMPANY OVERVIEW
16.10.2 REVENUE ANALYSIS
16.10.3 GEOGRAPHIC PRESENCE
16.10.4 PRODUCT PORTFOLIO
16.10.5 RECENT DEVELOPEMENTS
16.11 MEDTRONIC INC.
16.11.1 COMPANY OVERVIEW
16.11.2 REVENUE ANALYSIS
16.11.3 GEOGRAPHIC PRESENCE
16.11.4 PRODUCT PORTFOLIO
16.11.5 RECENT DEVELOPEMENTS
16.12 TERUMO MEDICAL CORPORATION
16.12.1 COMPANY OVERVIEW
16.12.2 REVENUE ANALYSIS
16.12.3 GEOGRAPHIC PRESENCE
16.12.4 PRODUCT PORTFOLIO
16.12.5 RECENT DEVELOPEMENTS
16.13 HITACHI MEDICAL SYSTEMS
16.13.1 COMPANY OVERVIEW
16.13.2 REVENUE ANALYSIS
16.13.3 GEOGRAPHIC PRESENCE
16.13.4 PRODUCT PORTFOLIO
16.13.5 RECENT DEVELOPEMENTS
16.14 SIEMENS HEALTHINEERS
16.14.1 COMPANY OVERVIEW
16.14.2 REVENUE ANALYSIS
16.14.3 GEOGRAPHIC PRESENCE
16.14.4 PRODUCT PORTFOLIO
16.14.5 RECENT DEVELOPEMENTS
16.15 KONINKLIJKE PHILIPS N.V
16.15.1 COMPANY OVERVIEW
16.15.2 REVENUE ANALYSIS
16.15.3 GEOGRAPHIC PRESENCE
16.15.4 PRODUCT PORTFOLIO
16.15.5 RECENT DEVELOPEMENTS
16.16 GE HEALTHCARE
16.16.1 COMPANY OVERVIEW
16.16.2 REVENUE ANALYSIS
16.16.3 GEOGRAPHIC PRESENCE
16.16.4 PRODUCT PORTFOLIO
16.16.5 RECENT DEVELOPEMENTS
16.17 CARESTREAM HEALTH INC.
16.17.1 COMPANY OVERVIEW
16.17.2 REVENUE ANALYSIS
16.17.3 GEOGRAPHIC PRESENCE
16.17.4 PRODUCT PORTFOLIO
16.17.5 RECENT DEVELOPEMENTS
16.18 SHIMADZU MEDICAL
16.18.1 COMPANY OVERVIEW
16.18.2 REVENUE ANALYSIS
16.18.3 GEOGRAPHIC PRESENCE
16.18.4 PRODUCT PORTFOLIO
16.18.5 RECENT DEVELOPEMENTS
16.19 CANON MEDICAL SYSTEMS CORPORATION
16.19.1 COMPANY OVERVIEW
16.19.2 GEOGRAPHIC PRESENCE
16.19.3 PRODUCT PORTFOLIO
16.19.4 RECENT DEVELOPEMENTS
16.20 CAMPO IMAGING
16.20.1 COMPANY OVERVIEW
16.20.2 REVENUE ANALYSIS
16.20.3 GEOGRAPHIC PRESENCE
16.20.4 PRODUCT PORTFOLIO
16.20.5 RECENT DEVELOPEMENTS
16.21 SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD
16.21.1 COMPANY OVERVIEW
16.21.2 REVENUE ANALYSIS
16.21.3 GEOGRAPHIC PRESENCE
16.21.4 PRODUCT PORTFOLIO
16.21.5 RECENT DEVELOPEMENTS
16.22 GALT MEDICAL CORP.
16.22.1 COMPANY OVERVIEW
16.22.2 GEOGRAPHIC PRESENCE
16.22.3 PRODUCT PORTFOLIO
16.22.4 RECENT DEVELOPEMENTS
16.23 SHENZHEN BASDA MEDICAL APPARATUS CO.,LTD
16.23.1 COMPANY OVERVIEW
16.23.2 REVENUE ANALYSIS
16.23.3 GEOGRAPHIC PRESENCE
16.23.4 PRODUCT PORTFOLIO
16.23.5 RECENT DEVELOPEMENTS
16.24 CORDIS
16.24.1 COMPANY OVERVIEW
16.24.2 REVENUE ANALYSIS
16.24.3 GEOGRAPHIC PRESENCE
16.24.4 PRODUCT PORTFOLIO
16.24.5 RECENT DEVELOPEMENTS
16.25 ANDRATEC
16.25.1 COMPANY OVERVIEW
16.25.2 REVENUE ANALYSIS
16.25.3 GEOGRAPHIC PRESENCE
16.25.4 PRODUCT PORTFOLIO
16.25.5 RECENT DEVELOPEMENTS
16.26 STRON MEDICAL
16.26.1 COMPANY OVERVIEW
16.26.2 REVENUE ANALYSIS
16.26.3 GEOGRAPHIC PRESENCE
16.26.4 PRODUCT PORTFOLIO
16.26.5 RECENT DEVELOPEMENTS
16.27 BALTON SP. Z O.O.
16.27.1 COMPANY OVERVIEW
16.27.2 REVENUE ANALYSIS
16.27.3 GEOGRAPHIC PRESENCE
16.27.4 PRODUCT PORTFOLIO
16.27.5 RECENT DEVELOPEMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST RELATED REPORTS
17. CONCLUSION
18. QUESTIONNAIRE
19. ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.