Global Interventional Oncology Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
2.86 Billion
USD
4.89 Billion
2024
2032
USD
2.86 Billion
USD
4.89 Billion
2024
2032
| 2025 –2032 | |
| USD 2.86 Billion | |
| USD 4.89 Billion | |
|
|
|
|
Interventional Oncology Devices Market Size
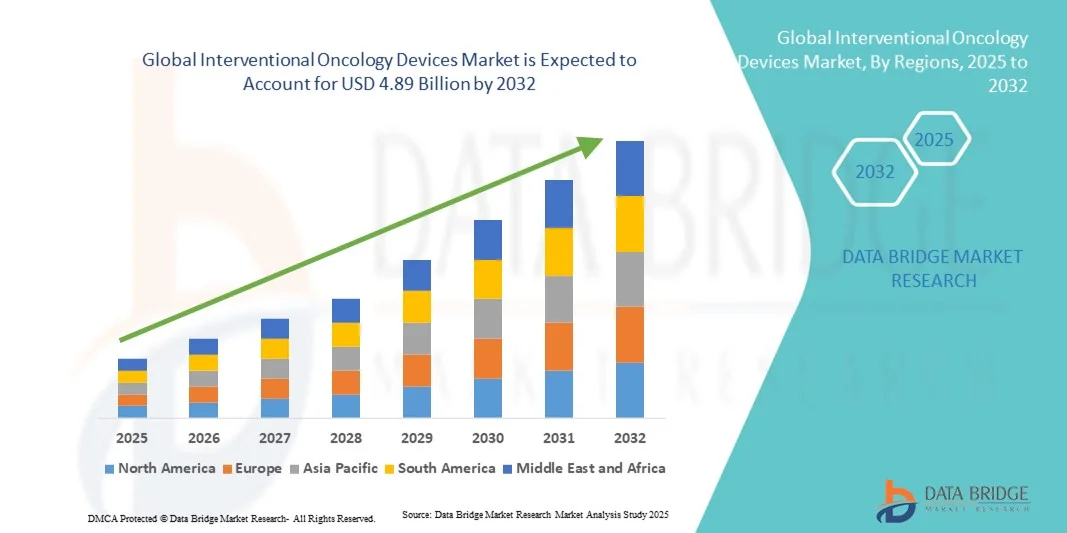
- The global interventional oncology devices market size was valued at USD 2.86 billion in 2024 and is expected to reach USD 4.89 billion by 2032, at a CAGR of 6.95% during the forecast period
- The market growth is largely fueled by the growing adoption and technological advancements in minimally invasive cancer treatment devices, enabling precise diagnosis and targeted therapy in both hospital and clinical settings. The integration of imaging guidance, robotics, and advanced catheter systems is enhancing the efficiency, accuracy, and safety of interventional oncology procedures
- Furthermore, rising demand for patient-centric, effective, and less invasive cancer treatment solutions is establishing interventional oncology devices as a preferred approach for tumor ablation, embolization, and localized drug delivery. Increasing awareness among healthcare providers and patients about the benefits of minimally invasive interventions is further driving adoption, thereby significantly boosting the industry’s growth
Interventional Oncology Devices Market Analysis
- Interventional oncology devices, including ablation systems, embolization devices, and image-guided catheters, are increasingly vital components of modern cancer care, enabling minimally invasive treatment, enhanced precision, and reduced recovery times in both hospital and specialty clinic settings
- The escalating demand for interventional oncology devices is primarily fueled by rising cancer prevalence, growing awareness of minimally invasive treatment options, technological advancements in imaging and catheter systems, and a preference for procedures that reduce hospital stay and improve patient outcomes
- North America dominated the interventional oncology devices market with the largest revenue share of 41.5% in 2024, characterized by advanced healthcare infrastructure, high healthcare expenditure, early adoption of innovative oncology devices, and a strong presence of key industry players. The U.S. experienced substantial growth in device installations across hospitals and cancer centers, driven by innovations in image-guided ablation, embolization, and robotic-assisted interventions
- Asia-Pacific is expected to be the fastest-growing region in the interventional oncology devices market during the forecast period, with a CAGR of 13.8% from 2025 to 2032, due to increasing urbanization, rising disposable incomes, government initiatives to improve cancer care, and expanding healthcare infrastructure in countries such as China and India
- The ablation devices segment dominated the largest market revenue share of 38.6% in 2024, driven by their proven efficacy in treating primary and metastatic tumors in organs such as the liver, kidney, and lungs
Report Scope and Interventional Oncology Devices Market Segmentation
|
Attributes |
Interventional Oncology Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Interventional Oncology Devices Market Trends
Advancements in Minimally Invasive and Image-Guided Therapies
- A significant and accelerating trend in the global interventional oncology devices market is the shift toward minimally invasive, image-guided, and precision-based therapies. These technologies are enhancing treatment accuracy, reducing procedural complications, and improving patient recovery times
- For instance, next-generation ablation systems, embolization devices, and radiation delivery platforms allow clinicians to target tumors more precisely while sparing surrounding healthy tissue. This trend is driving adoption in hospitals, specialty clinics, and ambulatory surgical centers
- Integration of imaging modalities such as CT, MRI, and ultrasound with interventional oncology devices enables real-time monitoring and improves procedural outcomes. Clinicians increasingly rely on these devices for complex tumor interventions in both early-stage and advanced cancers
- The trend toward personalized oncology treatment is also fueling growth, as devices can be tailored to patient-specific anatomy and tumor characteristics, increasing efficacy and patient satisfaction
- This move toward advanced, minimally invasive, and targeted therapies is reshaping treatment protocols and clinical expectations, prompting manufacturers to innovate devices that are safer, faster, and more efficient
- Consequently, key companies are focusing on developing devices with enhanced precision, ease of use, and broader application across multiple cancer types, which is expected to sustain growth in the market over the forecast period
Interventional Oncology Devices Market Dynamics
Driver
Growing Demand Due to Rising Cancer Incidence and Technological Advancements
- The increasing prevalence of cancer globally, coupled with rising awareness of minimally invasive treatment options, is a significant driver for the adoption of interventional oncology devices
- For instance, in April 2024, a leading medical device company launched a next-generation microwave ablation system, designed to improve precision in tumor targeting and reduce procedure times. Such innovations by key players are expected to drive the Interventional Oncology Devices industry growth in the forecast period
- As healthcare providers aim to enhance patient outcomes and reduce hospital stays, interventional oncology devices offer advanced features such as image-guided therapies, real-time monitoring, and minimally invasive approaches, providing a compelling alternative to conventional surgical methods
- Furthermore, the growing focus on early cancer detection, outpatient procedures, and personalized treatment options is increasing the demand for interventional oncology devices in hospitals, specialty clinics, and ambulatory surgical centers
- The convenience of minimally invasive procedures, reduced recovery times, and improved safety profiles are key factors propelling the adoption of interventional oncology devices across both developed and emerging markets. The trend toward outpatient oncology services and the increasing availability of clinically validated device options further contribute to market growth
Restraint/Challenge
High Costs and Regulatory Hurdles
- The relatively high cost of interventional oncology devices, compared to conventional surgical equipment, poses a significant challenge to broader market penetration. This can be a barrier for price-sensitive hospitals, clinics, and healthcare providers, especially in developing regions
- For instance, the high acquisition and maintenance costs of advanced ablation, embolization, and radiation delivery systems may delay adoption in smaller clinics or low-resource hospitals
- Navigating stringent regulatory requirements, including FDA and CE approvals, also presents a challenge for manufacturers aiming to launch new devices globally. Compliance with these regulations requires substantial investment in clinical trials, documentation, and post-market surveillance
- While prices are gradually decreasing due to technological advancements and increasing competition, the perceived premium for innovative oncology devices can still limit adoption, particularly among facilities with constrained budgets
- Overcoming these challenges through cost-effective device designs, flexible financing options, regulatory support, and clinician training programs will be vital for sustained growth of the interventional oncology devices market
Interventional Oncology Devices Market Scope
The market is segmented on the basis of product type, cancer type, and procedure.
- By Product Type
On the basis of product type, the Interventional Oncology Devices market is segmented into embolization devices, ablation devices, support devices, and next-generation devices. The ablation devices segment dominated the largest market revenue share of 38.6% in 2024, driven by their proven efficacy in treating primary and metastatic tumors in organs such as the liver, kidney, and lungs. Ablation devices, including radiofrequency, microwave, and cryoablation systems, provide minimally invasive options with shorter recovery times and reduced complications. High adoption rates in leading hospitals and specialty cancer centers enhance the segment’s market leadership. The segment benefits from continuous technological innovations improving precision and targeting accuracy. Integration with advanced imaging modalities such as CT, MRI, and ultrasound further boosts clinical outcomes. Physicians prefer ablation devices for localized tumor control without affecting surrounding healthy tissue. Increasing prevalence of cancer globally and the rising geriatric population support sustained demand. Regulatory approvals and reimbursement coverage in developed markets strengthen market dominance. Clinical preference for minimally invasive techniques reinforces the segment’s leading position. Continuous training and adoption of digital workflow in interventional oncology contribute to higher procedural adoption.
The next-generation devices segment is expected to witness the fastest CAGR of 14.5% from 2025 to 2032, driven by innovations in smart catheter systems, robotic-assisted interventions, and image-guided therapy platforms. These devices integrate advanced imaging, navigation, and automation to improve precision, reduce procedural time, and enhance patient safety. Growing R&D investments in smart interventional tools and AI-assisted planning fuel the segment’s rapid adoption. Increasing demand for personalized, minimally invasive treatment approaches encourages the use of next-generation devices in leading oncology centers. The segment also benefits from a shift toward outpatient-based procedures and shorter hospital stays. Rising awareness among clinicians about device efficacy and reduced complication rates drives uptake. Rapid adoption in emerging economies with improving healthcare infrastructure further supports CAGR growth. Strategic partnerships between device manufacturers and hospitals facilitate deployment. Availability of multi-functional, hybrid devices capable of combining ablation and embolization procedures enhances utility. Expansion of advanced procedural training programs accelerates clinician confidence and market penetration.
- By Cancer Type
On the basis of cancer type, the market is segmented into liver cancer, prostate cancer, breast cancer, lung cancer, bone cancer, kidney cancer, and others. The liver cancer segment accounted for the largest market revenue share of 29.8% in 2024, driven by the high incidence of hepatocellular carcinoma and metastatic liver tumors globally. Interventional oncology procedures like thermal ablation and transcatheter arterial chemoembolization (TACE) are standard-of-care for patients ineligible for surgery. Rising awareness of minimally invasive liver cancer treatments and increasing use of image-guided therapy contribute to segment dominance. Hospitals and specialty oncology centers are increasingly adopting these procedures due to high clinical efficacy. The segment benefits from technological improvements in real-time imaging, enabling precise targeting of tumors. Early diagnosis programs and screening initiatives in high-risk populations support increased procedure volumes. Regulatory approvals and guideline endorsements from medical societies reinforce adoption. Availability of specialized ablation and embolization devices tailored for liver anatomy drives preference. Rising prevalence of hepatitis B and C, major risk factors, ensures consistent demand. High reimbursement coverage in developed regions further strengthens market leadership.
The kidney cancer segment is expected to witness the fastest CAGR of 13.9% from 2025 to 2032, fueled by the increasing preference for minimally invasive procedures such as cryoablation and radiofrequency ablation over conventional surgery. Growth is supported by rising incidence of renal cell carcinoma, especially among the aging population. Technological advancements, including robotic-assisted ablation and real-time imaging integration, enhance procedural safety and efficacy. Expanding hospital infrastructure and adoption of outpatient-based interventional therapies further contribute to growth. Rising awareness about nephron-sparing treatments and reduced complication rates encourage clinician adoption. Increasing collaborations between device manufacturers and healthcare providers facilitate accessibility. Adoption of image-guided precision therapy reduces recovery time and supports patient preference for minimally invasive options. Government initiatives promoting early detection and minimally invasive care further fuel adoption. Growing adoption in emerging regions due to improved healthcare access contributes to CAGR. Research in combination therapies, integrating ablation with immunotherapy, boosts procedural appeal and market expansion.
- By Procedure
On the basis of procedure, the Interventional Oncology Devices market is segmented into thermal tumor ablation, non-thermal tumor ablation, transcatheter arterial chemoembolization (TACE), transcatheter arterial radioembolization/selective internal radiation therapy (TARE/SIRT), and transcatheter arterial embolization/bland embolization. The thermal tumor ablation segment dominated the largest market revenue share of 35.4% in 2024, driven by its wide clinical adoption for liver, kidney, and lung tumors. Techniques such as radiofrequency, microwave, and laser ablation are minimally invasive, cost-effective, and associated with high local tumor control rates. Integration with CT, MRI, and ultrasound imaging enhances accuracy and treatment efficacy. Shortened hospital stays and reduced post-operative complications improve patient satisfaction. High physician preference for established thermal ablation protocols supports segment dominance. Continuous innovation in needle design, energy delivery, and temperature monitoring enhances procedural outcomes. The segment benefits from strong reimbursement policies in developed countries. Increased use in combination with chemotherapy and immunotherapy expands clinical applications. Growth in oncology centers and specialist training programs reinforces adoption. Rising cancer prevalence and aging populations ensure sustained demand.
The TARE/SIRT segment is expected to witness the fastest CAGR of 14.2% from 2025 to 2032, fueled by increasing use of yttrium-90 and other radioisotopes for targeted internal radiation therapy. Adoption is rising due to high efficacy in treating unresectable liver tumors while sparing healthy tissue. Advancements in catheter delivery systems and imaging guidance improve procedural precision. Growth is supported by increasing availability of radioactive microspheres and regulatory approvals for newer isotopes. Hospitals and specialty oncology centers are expanding TARE/SIRT capabilities to meet rising demand. Physician awareness campaigns and clinical training programs facilitate rapid adoption. Minimally invasive nature and outpatient applicability make TARE/SIRT attractive to patients and providers. Integration with multidisciplinary care pathways enhances acceptance. Insurance coverage for targeted radiotherapy in key markets supports growth. Adoption in emerging markets increases with improved healthcare infrastructure.
Interventional Oncology Devices Market Regional Analysis
- North America dominated the interventional oncology devices market with the largest revenue share of 41.5% in 2024, driven by advanced healthcare infrastructure, high healthcare expenditure, early adoption of innovative oncology devices, and a strong presence of key industry players. The U.S. captured the largest share within the region, fueled by rapid installations of image-guided ablation, embolization, and robotic-assisted interventional oncology systems across hospitals and specialty cancer centers
- Increasing prevalence of cancer, rising demand for minimally invasive procedures, and strong reimbursement frameworks encourage widespread adoption. Integration of advanced imaging modalities with interventional devices enhances procedural precision and patient outcomes. Awareness programs, clinical training initiatives, and continuous investment in state-of-the-art oncology centers support market growth
- Regulatory approvals, favorable policies, and early adoption of technology strengthen the region’s leadership. The presence of major global manufacturers ensures availability of cutting-edge devices, while a skilled healthcare workforce facilitates high adoption rates
U.S. Interventional Oncology Devices Market Insight
The U.S. interventional oncology devices market captured the largest share within North America, contributing significantly to the regional dominance. The market accounted for a substantial portion of North America’s revenue in 2024, driven by rapid installations of image-guided ablation, embolization, and robotic-assisted interventional systems in hospitals and specialty cancer centers. High per capita healthcare spending, early technology adoption, and strong research and development infrastructure support market growth. The increasing preference for minimally invasive procedures, growing awareness of precision oncology, and robust reimbursement frameworks encourage adoption. Leading device manufacturers continue to introduce innovative solutions, while clinical training and awareness programs promote the use of advanced interventional procedures. Rising cancer prevalence and expansion of specialty oncology centers further boost market expansion.
Europe Interventional Oncology Devices Market Insight
The Europe interventional oncology devices market is projected to expand at a substantial CAGR throughout the forecast period, driven by growing investments in healthcare infrastructure, adoption of minimally invasive therapies, and increasing cancer prevalence. Germany, the U.K., and France are key markets witnessing high adoption of ablation and embolization devices due to improved clinical outcomes and shorter hospital stays. Germany leads the region with widespread use of image-guided and robotic-assisted interventions, while the U.K. benefits from expanding specialty oncology centers and well-established hospital networks. European growth is further supported by reimbursement policies, public health initiatives promoting precision oncology, and a focus on advanced procedural training. Rising awareness among clinicians and patients regarding minimally invasive treatment options encourages adoption across hospitals and clinics.
Germany Interventional Oncology Devices Market Insight
The Germany interventional oncology devices market is expected to expand at a considerable CAGR during the forecast period, supported by advanced healthcare infrastructure, increasing awareness of minimally invasive cancer treatments, and early adoption of next-generation interventional oncology devices. The country benefits from a strong presence of hospitals equipped with image-guided ablation and embolization systems, as well as robotic-assisted interventional technologies. Rising cancer incidence, particularly liver, lung, and kidney cancers, fuels demand for precise, minimally invasive therapies. Government initiatives promoting innovation and patient-centric care encourage adoption of cutting-edge interventional solutions. Strong reimbursement frameworks and well-developed healthcare networks facilitate the deployment of advanced devices in specialty oncology centers. Germany’s emphasis on sustainability and digital healthcare integration further drives the integration of high-tech interventional procedures. Hospitals and cancer centers are increasingly investing in hybrid suites combining imaging and interventional capabilities. Clinical training programs, along with public awareness campaigns, promote adoption of safe and effective cancer treatment solutions. The growing need for improved patient outcomes and reduced hospital stays continues to support market growth in the country.
U.K. Interventional Oncology Devices Market Insight
The U.K. interventional oncology devices market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the increasing trend of minimally invasive cancer therapies and adoption of advanced interventional technologies. Rising concerns regarding cancer prevalence, safety, and treatment efficiency encourage hospitals and specialty oncology centers to invest in image-guided ablation, embolization, and robotic-assisted devices. Well-established hospital networks and expanding specialty oncology centers contribute to market penetration. Public health initiatives and awareness programs highlighting the benefits of minimally invasive procedures support growth. The U.K. government’s healthcare policies and reimbursement frameworks further facilitate adoption of advanced interventional oncology devices. Rising investments in clinical research and training ensure proper utilization of sophisticated equipment. Growing patient preference for faster recovery times and outpatient procedures drives the demand for high-precision interventional solutions.
Asia-Pacific Interventional Oncology Devices Market Insight
Asia-Pacific interventional oncology devices market is expected to be the fastest-growing region in the Interventional Oncology Devices market during the forecast period, with a CAGR of 13.8% from 2025 to 2032, driven by increasing urbanization, rising disposable incomes, and expanding healthcare infrastructure. Countries such as China, India, and Japan are investing in modern oncology centers and advanced imaging-guided interventional devices. China accounted for the largest revenue share in the region in 2024, supported by rising cancer incidence, rapid urbanization, and government initiatives to improve cancer care. Japan’s market growth is propelled by a high-tech healthcare culture, aging population, and increasing adoption of minimally invasive therapies. India is witnessing growing adoption of embolization and ablation procedures due to expanding specialty cancer hospitals and cost-effective device availability.
China Interventional Oncology Devices Market Insight
The China interventional oncology devices market captured the largest share in Asia-Pacific in 2024, driven by the increasing incidence of liver and lung cancers, rapid urbanization, and investments in advanced oncology treatment centers. Availability of affordable devices, strong domestic manufacturing, and government cancer care initiatives further support market growth. Rising awareness of minimally invasive interventions and adoption of image-guided procedures are driving demand across hospitals and cancer centers.
Japan Interventional Oncology Devices Market Insight
The Japan interventional oncology devices market is growing steadily due to high adoption of image-guided ablation and embolization procedures. The country’s aging population, rising cancer prevalence, and preference for minimally invasive treatments are key growth drivers. Integration of robotic-assisted interventions and advanced imaging systems improves procedural accuracy, safety, and patient outcomes. Expanding specialty oncology centers and supportive reimbursement policies contribute to market expansion.
Interventional Oncology Devices Market Share
The Interventional Oncology Devices industry is primarily led by well-established companies, including:
• Medtronic (U.S.)
• Boston Scientific (U.S.)
• Johnson & Johnson (U.S.)
• Terumo Corporation (Japan)
• Cook Medical (U.S.)
• AngioDynamics (U.S.)
• Merit Medical Systems (U.S.)
• BTG International (U.K.)
• Philips Healthcare (Netherlands)
• Siemens Healthineers (Germany)
• Stryker (U.S.)
• MicroVention (U.S.)
• EndoChoice (U.S.)
• Halyard Health (U.S.)
• Canon Medical Systems (Japan)
• C.R. Bard (U.S.)
• Medico (India)
• Guerbet (France)
Latest Developments in Global Interventional Oncology Devices Market
- In July 2025, Varian (a part of Siemens Healthineers) introduced its IntelliBlate microwave ablation system in Europe, receiving CE‑mark approval for treatment of soft‑tissue tumours. The system integrates with Siemens’ imaging and planning tools to allow clinicians to plan, place, treat, monitor, and confirm ablation in a unified workflow
- In December 2023, China’s regulatory authority granted innovation approval to a cryoablation system and balloon‑type cryoablation catheter for tumour therapy as part of a batch of imported devices, highlighting growing regulatory momentum in interventional oncology in China
- In June 2023, Argon Medical launched its SuperCore Advantage semi‑automatic biopsy device, which is designed to secure up to ~35 % more tissue samples compared to existing systems, thereby improving diagnostic accuracy in interventional oncology settings.
- In April 2023, a market report on interventional oncology devices highlighted that increased private and government funding for tumour‑treatment devices, such as thermal ablation and TACE, is now a key growth driver for the segment. The report emphasised how embolisation devices and ablation systems are benefiting from greater R&D financing
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.