Global Intracranial Hematoma Drug Market
Market Size in USD Billion
CAGR :
% 
 USD
2.10 Billion
USD
4.10 Billion
2024
2032
USD
2.10 Billion
USD
4.10 Billion
2024
2032
| 2025 –2032 | |
| USD 2.10 Billion | |
| USD 4.10 Billion | |
|
|
|
|
Intracranial Hematoma Drug Market Size
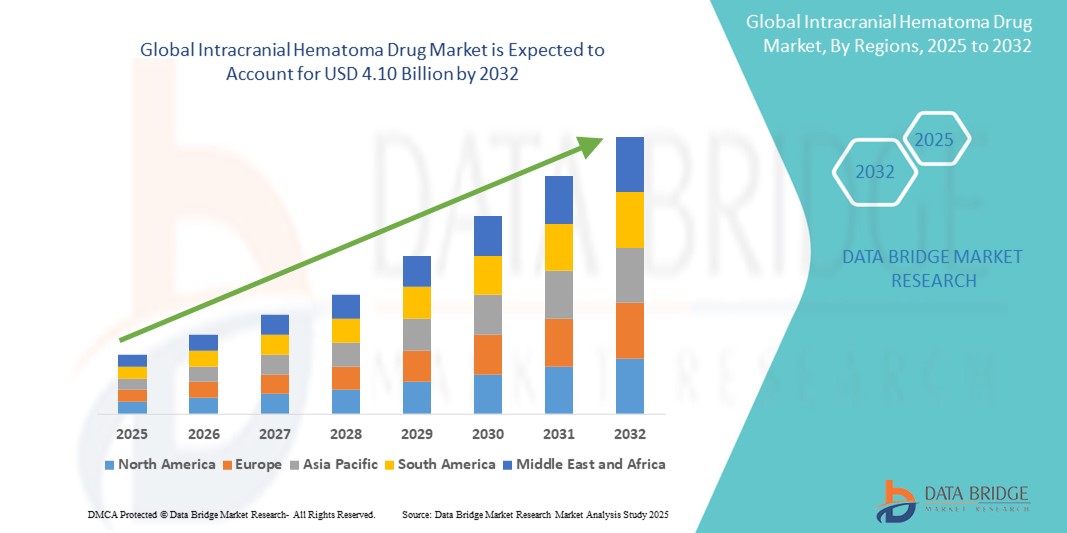
- The global Intracranial Hematoma Drug Market was valued at USD 2.10 billion in 2024 and is expected to reach USD 4.10 billion by 2032.
- During the forecast period of 2025 to 2032, the market is projected to grow at a CAGR of 6.6%, primarily driven by the rising incidence of traumatic brain injuries and growing clinical focus on rapid hematoma management.
- This growth is further propelled by factors such as advancements in neurotrauma therapeutics, increased awareness among emergency care providers, and rising demand for targeted drug therapies to reduce intracranial pressure and prevent secondary brain injuries.
Intracranial Hematoma Drug Market Analysis
- The global Intracranial Hematoma Drug Market is poised for substantial growth through 2032, registering a CAGR of 7.9% from 2025 to 2032.
- The increasing need for pharmacological options addressing both acute and long-term complications of intracranial hematomas, such as seizures, swelling, and inflammation, is boosting demand for anticoagulants, diuretics, and anticonvulsants.
- The market benefits from innovations in emergency neurocritical care, rising investment in brain trauma research, and growing emphasis on early intervention strategies.
- Growth in trauma care infrastructure, supportive government trauma management programs, and increasing clinical trials focusing on brain injury treatment protocols are expected to further enhance market performance, particularly in developing and low-to-middle-income countries
Report Scope and Intracranial Hematoma Drug Market Segmentation
|
Attributes |
Intracranial Hematoma Drug Market Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Global Intracranial Hematoma Drug Market Trends
“Shift Toward Targeted Neuroprotective and Anti-Inflammatory Therapies”
• A key trend in the global intracranial hematoma drug market is the focus on targeted therapies that address secondary brain injury mechanisms such as inflammation, oxidative stress, and neuronal apoptosis.
• There is a growing preference for neuroprotective agents, anticoagulant reversal drugs, and osmotic diuretics to manage acute hematoma symptoms and complications.
• For example, drugs like mannitol and hypertonic saline are widely used to reduce intracranial pressure and cerebral edema following hematoma.
• Novel delivery systems, including intranasal formulations and sustained-release injectables, are being developed to enhance brain penetration and rapid onset.
• The increasing incidence of traumatic brain injuries and advances in neuroimaging are driving demand for fast-acting pharmacologic interventions.
Market Dynamics
Driver
“Rising Incidence and Early Detection of Intracranial Hematomas”
The growing number of traumatic injuries and strokes globally, along with improved emergency diagnostic imaging, is increasing the need for immediate drug-based interventions.
Examples:
• According to the WHO, traumatic brain injuries are among the leading causes of mortality and disability worldwide, especially among young adults and the elderly.
• The widespread use of CT and MRI scans in emergency care is enhancing early identification and timely pharmacological treatment of intracranial hematomas.
“Government Support and Healthcare Infrastructure Development”
Public health initiatives and healthcare investments are fostering access to critical care treatments for brain injuries, including hematomas.
Examples:
• Governments in developing economies are expanding trauma centers and providing funding for neurological drug R&D.
• National stroke and trauma policies in countries such as India and Brazil are supporting access to neurosurgical drugs and emergency response systems.
Opportunity
“Development of Neuroprotective Agents and Adjunctive Therapies”
There is significant potential for innovative therapies that prevent secondary brain damage, improve neurological outcomes, and support long-term recovery.
Examples:
• Pharmaceutical companies are exploring anti-inflammatory agents, free radical scavengers, and calcium channel blockers for brain protection.
• Pipeline drugs targeting mitochondrial dysfunction and blood-brain barrier integrity are gaining clinical attention.
• Opportunities are also emerging for post-acute phase management, including drugs addressing cognitive deficits and seizures linked to hematoma complications.
Restraint/Challenge
“Limited Approved Therapies and Access Barriers in Resource-Limited Settings”
• Despite clinical needs, there are few approved drugs specifically indicated for intracranial hematoma management, limiting targeted treatment options.
• Delays in diagnosis and lack of access to neurocritical care facilities in low-resource settings hinder effective drug administration.
• The cost of intensive care drugs and neuroprotective agents remains prohibitive for many public health systems.
• Furthermore, drug delivery challenges, such as crossing the blood-brain barrier, and lack of clinical trials in diverse populations, impede broader adoption
Global Intracranial Hematoma Drug Market Scope
The market is segmented on the basis of drug class, therepy class, end-users, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
Drug Class |
|
|
Therepy type |
|
|
End-Users |
|
|
Distribution Channel |
|
Global Intracranial Hematoma Drug Market Regional Analysis
“United States is the Dominant Region in the Intracranial Hematoma Drug Market”
• Countries like the United States, Canada, and Germany dominate the market due to advanced neurology care infrastructure, high incidence of traumatic brain injuries (TBI), and robust clinical research in neurocritical medicine.
• Government initiatives for emergency care enhancement and healthcare reimbursement policies contribute to the market’s growth in these regions.
“Asia-Pacific and Latin America Show Promising Growth Potential”
• Growing awareness about traumatic brain injuries, improvements in diagnostic access, and expanding healthcare investment are fueling market expansion.
• India and Brazil are emerging as crucial markets driven by increased road accidents and improved neurosurgical care facilities.
• The use of cost-effective generic neuroprotective drugs and telemedicine-based follow-up care is enhancing drug accessibility in low-resource settings.
Global Intracranial Hematoma Drug Market Share
The competitive landscape provides a comprehensive overview of the leading market players. This includes company profiles, financial performance, R&D activities, product pipelines, international presence, production capabilities, strategic developments, strengths, weaknesses, and contributions to neurotrauma therapeutics.
The Major Market Leaders Operating in the Market Include:
• Pfizer Inc.
• F. Hoffmann-La Roche Ltd
• Teva Pharmaceutical Industries Ltd.
• Novartis AG
• Johnson & Johnson Services, Inc.
• Sanofi S.A.
• Bayer AG
• Bristol-Myers Squibb Company
• Mallinckrodt Pharmaceuticals
• Takeda Pharmaceutical Company Limited
• Zydus Lifesciences Limited
• Dr. Reddy’s Laboratories Ltd.
• Sun Pharmaceutical Industries Ltd.
• Aurobindo Pharma
• Amneal Pharmaceuticals, Inc.
Latest Developments in Global Intracranial Hematoma Drug Market
- In March 2023, Pfizer announced a Phase 2 trial for its anti-inflammatory agent aimed at reducing cerebral edema post-hematoma.
- In July 2022, Roche collaborated with a European neurology institute to co-develop biomarkers for drug responsiveness in intracranial hematoma patients.
- In 2024, South Korea’s health authority approved a novel neuroprotective formulation for acute hematoma management in emergency care units.
- The report also highlights emerging trends such as biomarker-driven drug targeting, AI in neuroimaging-based treatment stratification, and integration of neurorehabilitation with pharmacologic therapy.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.