Global Intraosseous Infusion Kits Market
Market Size in USD Billion
CAGR :
% 
 USD
873.06 Billion
USD
1,413.73 Billion
2025
2033
USD
873.06 Billion
USD
1,413.73 Billion
2025
2033
| 2026 –2033 | |
| USD 873.06 Billion | |
| USD 1,413.73 Billion | |
|
|
|
|
Intraosseous infusion kits Market Size
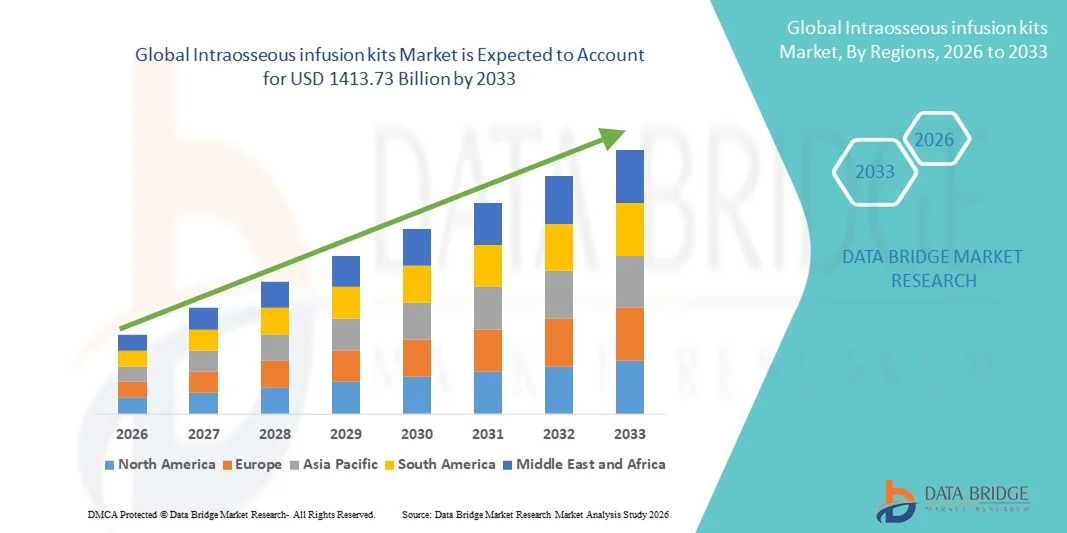
- The global intraosseous infusion kits market size was valued at USD 873.06 billion in 2025 and is expected to reach USD 1413.73 billion by 2033, at a CAGR of 6.21% during the forecast period
- The market growth is largely fueled by the growing adoption and technological advancements in emergency medical care and pre-hospital treatment systems, leading to increased demand for rapid vascular access solutions in both hospital and field settings
- Furthermore, rising demand from healthcare providers and emergency responders for fast, reliable, and easy-to-use vascular access devices is establishing intraosseous infusion kits as a preferred choice for critical care situations. These converging factors are accelerating the uptake of Intraosseous Infusion Kits solutions, thereby significantly boosting the industry's growth
Intraosseous infusion kits Market Analysis
- Intraosseous infusion kits, which provide rapid vascular access through the bone marrow for emergency fluid and medication administration, are increasingly essential in emergency medicine and critical care settings due to their ability to deliver fast and reliable access when traditional intravenous access is difficult or time-consuming
- The escalating demand for intraosseous infusion kits is primarily fueled by the growing incidence of trauma, cardiac arrest, and critical emergencies, along with increasing adoption of pre-hospital emergency care protocols and rising focus on improving survival outcomes through faster intervention
- North America dominated the intraosseous infusion kits market with the largest revenue share of 41.6% in 2025, supported by advanced emergency healthcare infrastructure, strong adoption of emergency medical services (EMS) protocols, high awareness among healthcare professionals, and widespread usage in hospitals and pre-hospital care settings, with the U.S. leading market demand
- Asia-Pacific is expected to be the fastest-growing region in the intraosseous infusion kits market during the forecast period, driven by increasing healthcare investments, rising incidence of emergencies and trauma cases, expanding emergency care facilities, and growing adoption of modern pre-hospital and hospital emergency care equipment across emerging economies
- The battery powered segment dominated the largest market revenue share of 52.6% in 2025, driven by the increasing preference for automated devices that ensure fast and accurate needle insertion
Report Scope and Intraosseous infusion kits Market Segmentation
|
Attributes |
Intraosseous infusion kits Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Intraosseous infusion kits Market Trends
Enhanced Operational Efficiency Through Advanced Intraosseous Access Solutions
- A significant and accelerating trend in the global intraosseous infusion kits market is the growing adoption of advanced intraosseous access devices that provide faster, safer, and more reliable vascular access in emergency and critical care settings
- For instance, emergency departments in North America and Europe are increasingly using automatic intraosseous devices that enable quick vascular access in trauma, cardiac arrest, and shock patients when traditional IV access is difficult
- The integration of improved needle design, pressure-resistant infusion lines, and standardized insertion protocols is enhancing the success rate of intraosseous access, reducing time-to-treatment, and improving patient outcomes
- In addition, the rise of pre-hospital care services and military emergency response units is accelerating the demand for portable and user-friendly intraosseous kits that can be used in challenging environments
- The trend toward minimally invasive emergency procedures and the need for rapid medication delivery during critical care situations are also driving innovation in intraosseous infusion technology
- This shift toward more reliable, efficient, and clinically effective intraosseous access systems is improving emergency response workflows and strengthening the overall critical care supply chain
Intraosseous infusion kits Market Dynamics
Driver
Increasing Emergency Care Procedures and Rising Incidence of Trauma and Cardiac Arrest
- The growing number of emergency cases and critical care incidents worldwide is a major driver for the global intraosseous infusion kits market
- For instance, rising road accidents, trauma cases, and emergency cardiac events in regions such as Asia Pacific and Latin America are increasing the need for rapid vascular access solutions
- Intraosseous infusion is now recognized as a vital alternative to intravenous access in emergency settings, especially for pediatric patients and those with collapsed veins
- Furthermore, expanding emergency medical services (EMS) and increased adoption of standardized emergency protocols are boosting the demand for intraosseous kits
- The rising focus on improving survival rates in critical care scenarios and the emphasis on timely administration of life-saving medications are driving hospitals and EMS providers to stock intraosseous infusion devices and consumables
- Overall, these factors are creating strong demand for intraosseous infusion kits across hospitals, ambulatory emergency units, and pre-hospital care services globally
Restraint/Challenge
High Cost of Advanced Devices and Limited Clinical Training
- High cost associated with advanced intraosseous devices and kits can limit adoption, particularly in low-income regions and smaller healthcare facilities
- For instance, budget constraints in rural hospitals and developing countries often restrict procurement of automatic intraosseous devices, leading to continued reliance on traditional IV access methods
- In addition, intraosseous access requires proper clinical training to ensure correct insertion and prevent complications such as infection or tissue damage
- Lack of adequate training among emergency care providers, paramedics, and nursing staff can hinder the effective use of intraosseous kits and reduce confidence in the procedure.
- Moreover, concerns related to procedural complications and lack of awareness about intraosseous access guidelines can slow adoption rates in some regions
- Addressing these challenges through cost-effective device options, training programs, and standardized clinical protocols will be essential for sustained growth of the global intraosseous infusion kits market
Intraosseous infusion kits Market Scope
The market is segmented on the basis of type, technology, route of administration, and end users.
- By Type
On the basis of type, the Global Intraosseous Infusion Kits market is segmented into FAST1 device, Dieckmann modified needle, BIG device, EZ-IO device, Jamshidi needle, FASTx device, and NIO. The EZ-IO device segment dominated the largest market revenue share of 35.4% in 2025, driven by its high reliability, ease of use, and rapid adoption in emergency care settings. EZ-IO devices are widely used by paramedics, emergency physicians, and trauma teams for quick vascular access in critical situations. The device’s battery-powered driver enables fast insertion and high success rates, even in challenging patient conditions. The strong brand recognition and extensive training support programs also contribute to market leadership. EZ-IO systems are compatible with a wide range of needles and patient demographics, increasing their clinical flexibility. Increasing incidence of cardiac arrest, trauma, and severe shock conditions boosts demand for rapid vascular access solutions. The device’s proven performance in prehospital and in-hospital emergency care further strengthens adoption. Regulatory approvals and widespread availability in major healthcare markets also support its dominance. Strong distribution networks and clinical endorsements reinforce market share. Overall, EZ-IO remains the most preferred intraosseous device in emergency care.
The BIG device segment is expected to witness the fastest CAGR of 18.9% from 2026 to 2033, driven by increasing adoption in prehospital and military emergency settings. The BIG device offers rapid intraosseous access without the need for electricity or batteries, making it highly suitable for field emergencies and resource-limited environments. Its simple manual mechanism allows quick training and usage by first responders. Growing focus on improving emergency response times and enhancing survival rates is fueling demand for the BIG device. The device’s portability and robustness support adoption in ambulances, disaster response teams, and remote healthcare facilities. Increasing use in pediatric and adult emergency care also contributes to growth. Rising awareness about the importance of intraosseous access in critical care is expanding the user base. In addition, strong support from emergency medical services and training programs is accelerating market penetration. The segment is expected to benefit from rising emergency care infrastructure in emerging markets. Overall, the BIG device segment is poised for rapid growth due to its operational advantages and versatility.
- By Technology
On the basis of technology, the Global Intraosseous Infusion Kits market is segmented into battery powered, manual, and impact driven. The battery powered segment dominated the largest market revenue share of 52.6% in 2025, driven by the increasing preference for automated devices that ensure fast and accurate needle insertion. Battery powered intraosseous devices offer high success rates and reduce procedural errors, which is critical during emergency situations. Healthcare providers prefer battery powered systems due to their reliability and ease of use in high-pressure environments. These devices are widely used in emergency departments, trauma centers, and prehospital settings. Strong brand presence and extensive clinical adoption support market leadership. Increasing investments in advanced emergency care equipment further support growth. The segment also benefits from continuous technological upgrades, such as improved battery life and ergonomic designs. High demand for rapid vascular access solutions in critical care drives adoption. Battery powered devices are commonly used in both adult and pediatric emergency care. Overall, battery powered technology remains the leading segment in the market.
The impact driven segment is expected to witness the fastest CAGR of 20.2% from 2026 to 2033, driven by growing adoption in prehospital and field emergency scenarios. Impact driven devices offer quick insertion without relying on batteries, making them ideal for use in remote areas and disaster situations. These devices provide consistent performance and require minimal training for emergency responders. Rising demand for portable and cost-effective intraosseous solutions is supporting market growth. Impact driven technology is increasingly used by military medical teams and emergency medical services. The devices are also gaining traction in developing regions due to their affordability and reliability. Increasing awareness about intraosseous access as a life-saving procedure is expanding the user base. The segment benefits from growing emergency care infrastructure and government initiatives to improve prehospital services. Overall, impact driven technology is expected to grow rapidly due to its operational advantages and cost-effectiveness.
- By Route of Administration
On the basis of route of administration, the Global Intraosseous Infusion Kits market is segmented into distal femur, sternum, distal and proximal tibia, and others. The distal and proximal tibia segment dominated the largest market revenue share of 46.8% in 2025, driven by its wide clinical acceptance and high success rates in both adult and pediatric patients. The tibial route is commonly preferred due to its easy anatomical access and minimal complications. Emergency care teams and trauma specialists often select the tibia for rapid intraosseous access during cardiac arrest and severe shock. Increasing incidence of trauma and emergency cases drives demand for tibial access devices. The segment benefits from strong clinical evidence supporting its efficacy and safety. Tibial access is widely taught in emergency medical training programs, further supporting adoption. The route is suitable for a broad patient population, including infants and adults. Increasing use in prehospital and in-hospital emergency settings reinforces market dominance. Availability of specialized tibial needles and compatible devices supports segment growth. Overall, tibial route remains the preferred choice for intraosseous infusion.
The sternum segment is expected to witness the fastest CAGR of 17.4% from 2026 to 2033, driven by growing adoption in adult emergency care and critical care settings. Sternal intraosseous access provides a stable and rapid route for drug administration and fluid resuscitation, particularly in cardiac arrest cases. The route is favored for its proximity to central circulation, enabling quick delivery of medications. Increasing focus on improving survival outcomes in cardiac emergencies supports demand for sternal access devices. The segment is also supported by advancements in sternal intraosseous needles and devices. Growing awareness among emergency physicians and critical care teams is boosting adoption. Sternal access is often used when peripheral venous access is difficult or delayed. The route is gaining traction in hospital emergency departments and intensive care units. Increasing clinical training and guidelines promoting sternal access further support growth. Overall, the sternal segment is expected to expand rapidly due to its clinical advantages.
- By End Users
On the basis of end users, the Global Intraosseous Infusion Kits market is segmented into hospitals, ambulatory surgical centres, and others. The hospitals segment dominated the largest market revenue share of 62.9% in 2025, driven by high demand for emergency care and critical care services. Hospitals are primary users of intraosseous infusion kits due to their requirement for rapid vascular access during trauma, cardiac arrest, and severe shock cases. Emergency departments and intensive care units rely on intraosseous devices to ensure timely medication administration. Increasing number of hospital admissions due to chronic diseases and emergency cases supports segment dominance. Hospitals also have greater access to trained medical professionals and advanced emergency care infrastructure. The segment benefits from continuous investments in emergency and trauma care facilities. Rising focus on patient safety and improved survival outcomes supports adoption. Hospitals also drive demand through procurement of standardized intraosseous systems and training programs. Overall, hospitals remain the largest end users in the market.
The ambulatory surgical centres segment is expected to witness the fastest CAGR of 16.8% from 2026 to 2033, driven by increasing number of outpatient surgeries and expansion of ASCs globally. Ambulatory surgical centres are increasingly equipped with emergency response systems and require rapid vascular access solutions for unexpected critical situations. Growing adoption of minimally invasive procedures and same-day surgeries is increasing demand for intraosseous devices in ASCs. The segment benefits from rising investments in outpatient care facilities and expansion of surgical centers in developing regions. Increasing awareness among ASC staff regarding emergency protocols supports adoption. ASCs are also adopting portable and easy-to-use intraosseous devices for quick access. The growing preference for cost-effective outpatient care models further supports market growth. Overall, ASCs are expected to witness rapid growth due to rising outpatient surgical volumes and emergency preparedness.
Intraosseous infusion kits Market Regional Analysis
- North America dominated the intraosseous infusion kits market with the largest revenue share of 41.6% in 2025, supported by advanced emergency healthcare infrastructure, strong adoption of emergency medical services (EMS) protocols, high awareness among healthcare professionals, and widespread usage in hospitals and pre-hospital care settings, with the U.S. leading market demand. The region’s well-established EMS networks and trauma care facilities drive the adoption of intraosseous access devices for rapid vascular access in critical situations
- The presence of key market players and strong distribution networks also support high market penetration. Furthermore, continuous investments in emergency care equipment and training programs contribute to increased adoption. The demand for faster and more reliable vascular access solutions in ambulances and emergency departments is rising, which supports market growth
- North American healthcare providers increasingly prefer modern intraosseous systems due to their higher success rates and ease of use. In addition, the growing number of trauma and cardiac arrest cases further fuels demand. Overall, North America remains the leading market for intraosseous infusion kits due to strong clinical acceptance and advanced emergency care infrastructure
U.S. Intraosseous infusion kits Market Insight
The U.S. intraosseous infusion kits market captured the largest revenue share within North America in 2025, fueled by advanced healthcare infrastructure, high emergency care spending, and strong adoption of EMS protocols. The country’s extensive pre-hospital care network supports widespread usage of intraosseous devices for rapid vascular access in trauma and cardiac emergencies. In addition, increasing training programs for paramedics and emergency clinicians enhance device adoption. The U.S. market also benefits from high awareness of advanced emergency care technologies and strong R&D investments. Growing demand for efficient and quick access devices in hospitals and ambulatory care settings further strengthens market growth. Furthermore, the rising incidence of emergencies and trauma cases contributes to higher demand for intraosseous infusion kits. Overall, the U.S. remains the most significant market globally due to its mature healthcare ecosystem and strong clinical adoption.
Europe Intraosseous infusion kits Market Insight
The Europe intraosseous infusion kits market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing healthcare digitalization, improving emergency care infrastructure, and rising prevalence of trauma and critical care cases. Governments across Europe are strengthening emergency care services and investing in advanced medical devices to improve patient outcomes. The growing emphasis on improving pre-hospital care and reducing mortality in emergency situations is supporting market growth. In addition, strong clinical awareness and adoption of intraosseous access devices in hospitals and ambulance services further boost demand. Europe also benefits from increasing training programs for healthcare professionals and rising adoption of modern emergency care equipment. Overall, the region shows steady growth driven by improving healthcare infrastructure and rising demand for efficient emergency vascular access solutions.
U.K. Intraosseous infusion kits Market Insight
The U.K. intraosseous infusion kits market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing emergency care modernization and rising adoption of advanced medical devices. The U.K. government’s focus on improving emergency response and critical care outcomes supports the adoption of intraosseous access systems. In addition, growing training programs for paramedics and emergency clinicians enhance awareness and use of these devices. The presence of leading healthcare providers and strong emergency care infrastructure also supports market expansion. Increasing incidents of trauma and cardiac emergencies further drive demand. Overall, the U.K. market is expected to grow steadily due to rising investments in emergency care and increasing adoption of modern vascular access solutions.
Germany Intraosseous infusion kits Market Insight
The Germany intraosseous infusion kits market is expected to expand at a considerable CAGR during the forecast period, fueled by strong healthcare infrastructure, increasing emergency care modernization, and rising awareness of intraosseous access devices. Germany’s focus on improving pre-hospital and hospital emergency care services supports the adoption of modern intraosseous systems. The country also benefits from advanced clinical training programs and increasing investments in emergency care equipment. In addition, the growing number of trauma cases and aging population contribute to higher demand. Germany’s strong medical device manufacturing and innovation ecosystem further supports market growth. Overall, the market is expected to grow steadily due to strong clinical acceptance and healthcare investments.
Asia-Pacific Intraosseous infusion kits Market Insight
The Asia-Pacific intraosseous infusion kits market is expected to be the fastest-growing region during the forecast period, driven by increasing healthcare investments, rising incidence of emergencies and trauma cases, expanding emergency care facilities, and growing adoption of modern pre-hospital and hospital emergency care equipment across emerging economies. Rapid expansion of healthcare infrastructure in countries such as China and India is supporting market growth. The rising prevalence of accidents, cardiac emergencies, and critical illnesses is increasing demand for quick vascular access devices. In addition, growing adoption of EMS protocols and training programs for emergency clinicians are boosting awareness and use of intraosseous infusion kits. Emerging economies are also witnessing increased government initiatives to improve emergency care services and expand access to advanced medical devices. Overall, APAC is projected to grow rapidly due to increasing healthcare spending and expanding emergency care capabilities.
Japan Intraosseous infusion kits Market Insight
The Japan intraosseous infusion kits market is gaining momentum due to increasing healthcare investments, rising emergency care modernization, and high demand for advanced medical devices. Japan’s strong healthcare infrastructure and focus on improving emergency response outcomes support the adoption of intraosseous systems. In addition, the aging population and increasing incidence of critical illnesses contribute to higher demand. Growing awareness among healthcare professionals and adoption of modern EMS protocols also drive growth. Overall, Japan remains a key market in APAC due to its advanced healthcare ecosystem.
China Intraosseous infusion kits Market Insight
The China intraosseous infusion kits market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to increasing healthcare infrastructure investments, expanding emergency care facilities, and growing incidence of trauma and critical care cases. The country’s expanding healthcare system and strong government initiatives to improve emergency response and critical care outcomes are supporting market growth. Increasing adoption of modern medical devices and rising EMS capabilities further boost demand. In addition, growing awareness among healthcare professionals and rising training programs support intraosseous device adoption. Overall, China remains the leading market in APAC due to its large patient base and expanding emergency care services.
Intraosseous infusion kits Market Share
The Intraosseous infusion kits industry is primarily led by well-established companies, including:
• Teleflex (U.S.)
• ICU Medical (U.S.)
• Vidacare (U.S.)
• Teleflex (U.S.)
• Smiths Medical (U.S.)
• Cardinal Health (U.S.)
• Becton Dickinson (U.S.)
• B. Braun Melsungen (Germany)
• Vygon (France)
• Teleflex (Ireland)
• H&H Medical Corporation (U.S.)
• HMD Medical (U.S.)
• C.R. Bard (U.S.)
• H. P. Medical (China)
• Jiangsu Kindstar (China)
• Shenzhen Dapeng Medical (China)
• NIO Medical (Germany)
Latest Developments in Global Intraosseous infusion kits Market
- In March 2023, the intraosseous infusion devices market was forecast to grow significantly at a CAGR of around 6.9% from 2023 to 2033, driven by rising emergency care needs and technological advancements in intraosseous systems used when IV access is challenging, underscoring the expanding role of IO devices in critical medical scenarios
- In July 2023, industry studies highlighted emerging trends in the global intraosseous infusion devices market, including increased adoption in emergency care settings and a focus on product launches and collaborations among leading market players to expand portfolio offerings and global presence
- In May 2024, Teleflex expanded its intraosseous vascular access portfolio with the launch of the new Arrow EZ-IO Intraosseous Access Procedure Tray, the first FDA-cleared sterile, single-use tray designed to streamline clinician workflow and improve accessibility of IO devices in sterile fields across hospital and emergency environments
- In October 2024, BD (Becton, Dickinson and Company) launched its enhanced BD® Intraosseous Vascular Access System, featuring integrated passive needle tip safety to protect care providers and patients and enabling rapid delivery of fluids or medication in critical care situations where IV access may be delayed or difficult
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.