Global Ivd Reagents Market
Market Size in USD Billion
CAGR :
% 
 USD
54.89 Billion
USD
99.85 Billion
2024
2032
USD
54.89 Billion
USD
99.85 Billion
2024
2032
| 2025 –2032 | |
| USD 54.89 Billion | |
| USD 99.85 Billion | |
|
|
|
|
IVD Reagents Market Size
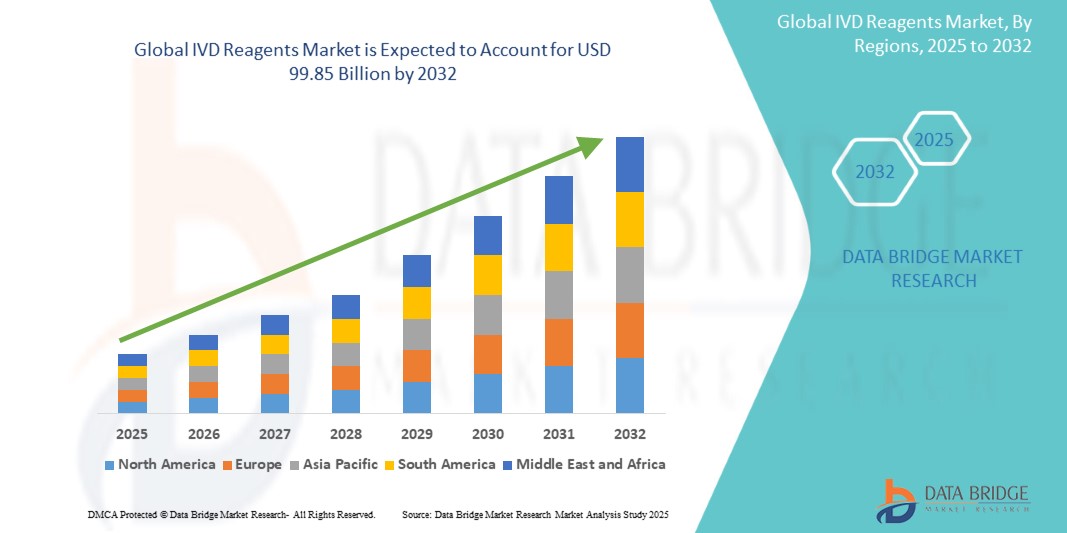
- The global IVD Reagents market was valued at USD 54.89 billion in 2024 and is expected to reach USD 99.85 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 4.60% primarily driven by the increasing demand for early disease detection and personalized medicine
- This growth is driven by factors such as the rising prevalence of chronic diseases, advancements in technology, and the growing focus on preventive healthcare
IVD Reagents Market Analysis
- In vitro diagnostics (IVD) reagents are substances or compounds used in laboratory tests to detect and diagnose diseases or conditions in a sample (such as blood, urine, or tissue) taken from a patient
- The in vitro diagnostics reagents market is expanding, with increasing demand for diagnostic testing in healthcare facilities.
- For instance, The COVID-19 pandemic significantly boosted the demand for diagnostic reagents, as seen with the surge in PCR and antigen test kits
- Advancements in diagnostic technologies are boosting the market, such as the integration of Artificial Intelligence and automation in diagnostic labs
- For instances, Automated testing systems such as Roche’s Cobas and Abbott’s Alinity have enhanced diagnostic efficiency and accuracy
- The growing focus on personalized medicine is driving demand for more precise and targeted diagnostics. Companies such as Illumina and Thermo Fisher Scientific are leading in next-generation sequencing technologies, which are vital for personalized healthcare
- Routine screening and disease monitoring, particularly for chronic diseases such as diabetes, continue to expand the use of diagnostic reagents. In real-time, millions use home testing kits, such as Abbott's FreeStyle Libre, to manage diabetes
- Key players are investing heavily in R&D to develop new reagents and diagnostic tests
- For instance, Siemens Healthineers' continuous development of reagents for immunoassays is a prime instance of market innovation
Report Scope and IVD Reagents Market Segmentation
|
Attributes |
IVD Reagents Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
IVD Reagents Market Trends
“Increasing Use of Molecular Diagnostics”
- The increasing use of molecular diagnostics is transforming the healthcare landscape, as it enables accurate and early detection of diseases at the genetic level. PCR-based tests became vital for detecting COVID-19 infections globally
- Technologies such as polymerase chain reaction and next-generation sequencing are being adopted more widely for disease diagnosis. In cancer, molecular diagnostics are used to identify specific mutations, guiding treatment decisions, as seen with tests such as FoundationOne for identifying genetic mutations in tumors
- Molecular diagnostics are crucial in personalized medicine, allowing treatments to be tailored to the individual's genetic makeup
- For instance, Genetic tests such as BRCA1 and BRCA2 are used to assess breast cancer risk, which can influence decisions on preventive care and treatment plans
- The demand for molecular diagnostics is expanding in infectious disease detection. Real-time PCR tests have become a standard for diagnosing diseases such as tuberculosis and HIV, allowing for rapid and accurate results that improve patient outcomes
- Advances in molecular diagnostics are also accelerating the development of multiplex testing, enabling the simultaneous detection of multiple pathogens.
- For instance, The BioFire FilmArray system can identify a wide range of pathogens in a single test, streamlining diagnostic processes
IVD Reagents Market Dynamics
Driver
“Increasing Demand for Early Disease Detection and Personalized Medicine”
- The demand for early disease detection is increasing, especially in critical areas such as oncology, cardiology, and infectious diseases, where early diagnosis can drastically improve patient survival.
- For instance, Early-stage detection of breast cancer through mammography or genetic testing for BRCA mutations has led to better treatment outcomes and survival rates
- Molecular diagnostics and genetic testing are transforming personalized medicine, helping healthcare providers tailor treatment plans based on individual genetic profiles.
- For instance, Genetic testing in oncology, such as FoundationOne, helps doctors identify the most effective therapies based on a tumor’s genetic mutations, leading to more precise and successful treatments
- The growing trend of preventive healthcare has spurred an increase in routine screenings, enhancing early detection and proactive disease management. Blood glucose tests and cholesterol screenings are now part of regular check-ups, helping identify risks for chronic conditions such as diabetes and heart disease before they become critical
- The shift towards personalized medicine has led to advancements in diagnostic reagents that offer a more tailored approach to treatment
- For instance, Pharmacogenomic testing helps doctors determine how patients will respond to certain drugs, reducing adverse drug reactions and optimizing treatment strategies
Opportunity
“Growth in Point-of-Care Testing and Home Diagnostics”
- The growing trend of point-of-care testing is increasing demand for quick, accessible, and reliable diagnostic solutions.
- For instance, During the COVID-19 pandemic, companies such as Abbott and Roche developed rapid antigen and PCR tests that could be used in non-laboratory settings, such as at home or in community health centers, allowing for fast diagnosis and timely intervention
- Point-of-care testing reduces patient wait times and enables healthcare providers to make prompt clinical decisions, improving patient outcomes
- For instance, Portable devices such as Roche’s cobas Liat System allow clinicians to test for infectious diseases such as flu and strep throat in a matter of minutes, supporting faster treatment decisions
- The increasing demand for home diagnostics, especially for chronic disease management, is driving market growth
- For instance, Is the use of home glucose monitoring systems such as the FreeStyle Libre from Abbott, which enables patients with diabetes to monitor their blood sugar levels and make immediate adjustments to their treatment plans
- Point-of-care tests for infectious diseases are especially beneficial for remote areas or underserved populations
- For instance, Malaria diagnostic kits, such as those developed by SD Biosensor, allow healthcare workers in low-resource settings to quickly and accurately diagnose malaria without needing advanced laboratory infrastructure
- The development of innovative at-home diagnostic kits is expanding the market for self-testing
- For instance, The Everlywell platform offers a range of at-home testing options, including for food sensitivity, hormones, and sexually transmitted infections, making healthcare more accessible and personalized
Restraint/Challenge
“Regulatory and Quality Control Issues”
- Regulatory and quality control issues pose a significant challenge for the in vitro diagnostics reagents market, as diagnostic reagents must comply with stringent regulations from authorities such as the Food and Drug Administration in the U.S. or the European Medicines Agency in Europe.
- For instance, The regulatory approval process for new diagnostic tests often involves extensive clinical trials and documentation, which can delay product launches and increase costs
- The complexity of these regulations varies across different regions, making it difficult for companies to navigate international markets.
- For instance, Diagnostic reagents that are approved in one region may require additional testing or modifications to meet the regulatory requirements in another region, creating hurdles for companies seeking global distribution
- Smaller companies or new entrants face additional challenges when meeting regulatory requirements, as the process can be resource-intensive. This often limits their ability to compete with larger, established companies that have the resources to navigate the regulatory landscape efficiently
- Continuous regulatory updates and changes in standards can further complicate the process.
- For instance, The implementation of stricter regulations on medical devices or reagents may force companies to modify their existing products or manufacturing processes, incurring additional expenses and delays in getting products to market
- Quality control is essential in maintaining the performance and reliability of diagnostic reagents. Any discrepancies, such as batch-to-batch variation or contamination, can undermine the accuracy of diagnostic tests, leading to potential product recalls, loss of consumer trust, and significant financial damage. Maintaining strict quality assurance processes is crucial to avoid such risks
IVD Reagents Market Scope
The market is segmented on the basis of type of reagents, technology, application, test type, and end-user.
|
Segmentation |
Sub-Segmentation |
|
By Type of Reagents |
|
|
By Technology |
|
|
By Application |
|
|
By Test Type |
|
|
By End-User |
|
IVD Reagents Market Regional Analysis
“North America is the Dominant Region in the IVD Reagents Market”
- North America is dominating the IVD Reagents market due to its well-established hospitals and diagnostic laboratories, the region is well-equipped to conduct a high volume of diagnostic testing, enhancing the demand for reagents
- The U.S., in particular, is home to leading players such as Abbott, Roche, and Thermo Fisher Scientific, which significantly contribute to the region’s market dominance
- North America benefits from favourable reimbursement policies, which make diagnostic tests more accessible and affordable for patients, further fuelling market growth
- The high demand for early disease detection, personalized medicine, and point-of-care testing also boosts the adoption of in vitro diagnostic reagents in the region. Furthermore, the ongoing advancements in molecular diagnostics, genetic testing, and automation technologies continue to support the demand for these reagents in hospitals, clinics, and research labs
- Another key factor contributing to North America's dominance is the high prevalence of chronic diseases, including cardiovascular conditions, diabetes, and cancer. This growing disease burden has led to a greater focus on routine diagnostic testing, increasing the need for diagnostic reagents. As a result, North America remains the dominant region in the in vitro diagnostics reagents market, both in terms of market share and technological advancements
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is expected to witness the highest growth rate in the IVD Reagents market, driven by rapidly expanding healthcare infrastructure, increasing healthcare awareness, and the rising prevalence of chronic diseases such as diabetes, cardiovascular diseases, and infections
- Major cities in North India, such as Delhi, Noida, Chandigarh, and Jaipur, have become prominent healthcare hubs, driving demand for diagnostic reagents. These cities are home to numerous hospitals, diagnostic laboratories, and healthcare centers, which have significantly increased the demand for diagnostic testing
- The increasing adoption of point-of-care testing, home diagnostics, and advanced molecular diagnostic technologies in these cities is further contributing to the market’s growth. The growing middle-class population in North India, combined with an increasing focus on preventive healthcare, has led to a higher demand for routine diagnostic tests, including cholesterol screenings, blood glucose tests, and cancer markers
- The region’s population density and urbanization also play a key role in accelerating the demand for in vitro diagnostics, with more people seeking immediate and accessible healthcare solutions. As a result, North India has emerged as the fastest-growing region in the market, with continuous growth expected due to the expanding healthcare infrastructure and increasing disease awareness
IVD Reagents Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Danaher Corporation (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Chembio Diagnostics, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Sysmex Corporation (Japan)
- Surmodics, Inc. (U.S.)
- Abbott (U.S.)
- QIAGEN N.V. (Netherlands)
- Merck KGaA (Germany)
- Agilent Technologies, Inc. (U.S.)
- MEDICAL & BIOLOGICAL LABORATORIES CO., LTD. (Tokyo)
- Siemens Healthineers AG (Germany)
- Revvity (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- DiaSorin S.p.A (Italy)
- Canvax (Spain)
- Illumina, Inc. (U.S.)
- Grifols, S.A. (Spain)
- Prestige Diagnostics (Ireland)
Latest Developments in Global IVD Reagents Market
- In December 2023, Roche entered into a definitive agreement to acquire LumiraDx's Point of Care technology. This acquisition involves a platform that integrates multiple diagnostic modalities on a single instrument, enabling faster, more affordable, and accessible testing in decentralized healthcare settings. The technology will enhance Roche’s existing diagnostics portfolio, spanning across immunoassay, clinical chemistry, and molecular tests. By incorporating LumiraDx's innovations, Roche aims to improve patient access to timely and accurate diagnostics in various settings, such as home care, pharmacies, and general practices. This acquisition is expected to expand Roche’s point-of-care solutions and drive market growth in decentralized diagnostics
- In July 2022, Thermo Fisher Scientific showcased new clinical and research lab solutions at AACC 2022. The company introduced innovative platforms and technologies designed to improve diagnostic development and laboratory productivity. These solutions, including advanced testing kits and automation systems, aim to minimize hands-on time and accelerate time-to-results, enhancing efficiency in clinical diagnostics. The impact on the market includes a significant boost in the adoption of rapid, high-precision diagnostics, particularly in infectious diseases and autoimmune testing. This development positions Thermo Fisher as a leader in the evolving diagnostics sector, supporting improved patient outcomes globally
- In May 2022, Hologic received the European CE mark for two new molecular assays—the Panther Fusion EBV Quant Assay and Panther Fusion BKV Quant Assay—designed for transplant patients. These assays quantify the viral load of Epstein-Barr virus and BK virus in whole blood, plasma, and urine samples. By aiding in the diagnosis and management of solid organ and stem cell transplant patients, these assays provide critical tools for monitoring infections in immunocompromised individuals. The new assays will enhance diagnostic capabilities on the Panther Fusion system, improving patient outcomes and driving market growth in transplant diagnostics
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.