Global Joubert Syndrome Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
8.25 Billion
USD
11.42 Billion
2024
2032
USD
8.25 Billion
USD
11.42 Billion
2024
2032
| 2025 –2032 | |
| USD 8.25 Billion | |
| USD 11.42 Billion | |
|
|
|
|
Joubert Syndrome Treatment Market Size
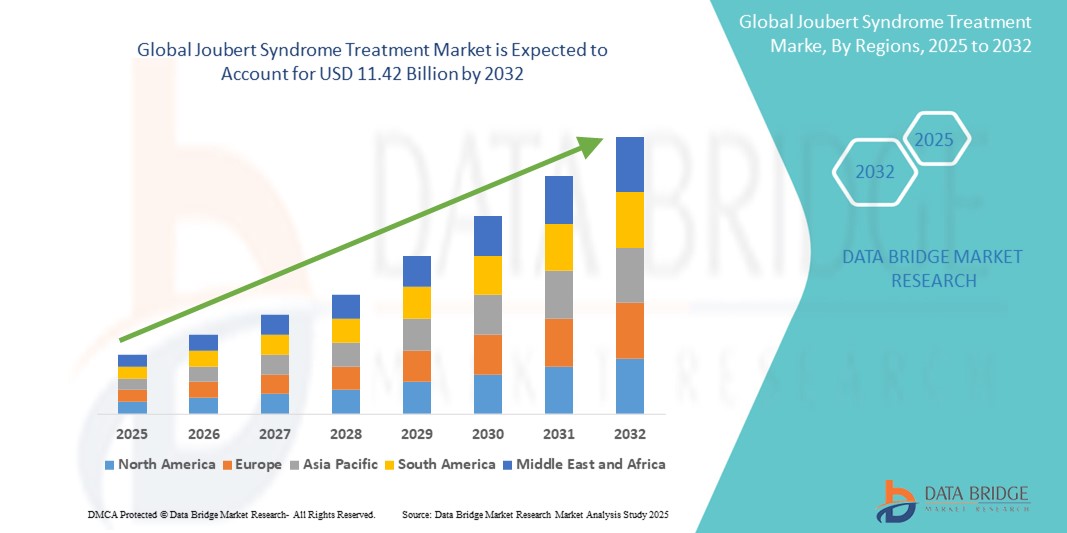
- The global joubert syndrome treatment market size was valued at USD 8.25 billion in 2024 and is expected to reach USD 11.42 billion by 2032, at a CAGR of 4.50% during the forecast period
- This growth is driven by rising awareness of rare genetic disorders, advances in molecular diagnostics, and supportive care availability in specialized centers
Joubert Syndrome Treatment Market Analysis
- Joubert syndrome is a rare genetic disorder affecting the cerebellum and brainstem, leading to developmental delays, hypotonia, and breathing abnormalities. Current treatment focuses on symptom relief, physiotherapy, respiratory support, and investigational gene therapies
- The market is supported by improving access to pediatric neurology services, increased genetic counseling, and a growing pipeline of experimental treatments
- North America dominates the Joubert Syndrome Treatment market with a share of approximately 41.6%, driven by active rare disease registries, early diagnostic initiatives, and presence of specialized care centers
- Asia-Pacific is projected to register the highest CAGR during the forecast period due to increasing government initiatives in rare disease management and improvements in neonatal screening
- The physical therapy segment is expected to account for the highest growth rate and largest market share of 42.28%, driven by its essential role in enhancing neuromotor development and managing hypotonia, a core symptom of joubert syndrome
Report Scope and Joubert Syndrome Treatment Market Segmentation
|
Attributes |
Joubert Syndrome Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Joubert Syndrome Treatment Market Trends
"Advances in Early Diagnosis and Molecular Screening for Joubert Syndrome"
- Progress in whole-exome sequencing (WES) and neuroimaging has significantly improved the accuracy of Joubert Syndrome (JS) diagnosis, enabling identification of causative mutations in over 35 genes, including CEP290, TMEM67, and AHI1
- The characteristic “molar tooth sign” (MTS) on MRI, combined with WES, confirms diagnoses in 62–97% of cases, even in clinically heterogeneous presentations
- Early detection, often in infancy, facilitates timely interventions like physical therapy and respiratory support, reducing long-term disabilities such as ataxia and developmental delay. Initiatives like the NIH’s Rare Disease Clinical Research Network (RDCRN) are enhancing awareness, funding studies, and improving access to genetic counseling, particularly for underserved populations
- For instance, a 2024 NIH RDCRN study used WES to diagnose JS in 95% of 50 pediatric patients, identifying novel CPLANE1 variants and enabling early occupational therapy to improve motor outcomes
- Advances in early diagnosis and molecular screening are transforming the Joubert Syndrome treatment landscape by enabling precise, timely interventions and driving research into targeted therapies
- The integration of WES, advanced neuroimaging, and NIH RDCRN initiatives is revolutionizing early diagnosis of Joubert Syndrome, enhancing patient outcomes and fueling market growth through improved intervention strategies
Joubert Syndrome Treatment Market Dynamics
Driver
"Improved Pediatric Neurology Infrastructure and Diagnostic Capabilities"
- The expansion of pediatric neurology units, coupled with enhanced neurogenetic services, has significantly increased Joubert Syndrome diagnosis rates
- Advanced diagnostic tools, such as high-resolution MRI and WES, are now more accessible in specialized centers, enabling identification of the MTS and mutations in genes like CC2D2A and NPHP1
- These improvements facilitate early referrals to multidisciplinary teams, including neurologists, geneticists, and ophthalmologists, driving demand for supportive therapies like speech and physical therapy
- For instance, in 2024, the European Reference Network for Rare Neurological Diseases reported a 20% increase in JS referrals due to new pediatric neurology units in Germany and France, improving diagnostic yield
- Telemedicine and training programs for pediatricians are further enhancing diagnostic capabilities in remote areas.Increased diagnosis rates are boosting treatment uptake, including assistive devices and rehabilitation services, expanding the market
- Improved pediatric neurology infrastructure and diagnostic capabilities are a key driver for the Joubert Syndrome treatment market, enabling earlier and more accurate diagnoses to support comprehensive care
- Enhanced pediatric neurology infrastructure and diagnostic tools are accelerating Joubert Syndrome diagnoses, driving treatment demand and fostering market growth through specialized care
Opportunity
“Gene Therapy Pipeline and Clinical Research Growth "
- The growing pipeline of gene therapies targeting JS-related mutations, such as TMEM67, CEP290, and ARL13B, offers long-term therapeutic potential
- Preclinical studies using CRISPR and AAV-based approaches are showing promise in restoring ciliary function, addressing the root cause of JS as a ciliopathy
- The NIH RDCRN and industry-academia collaborations are funding Phase I/II trials, while orphan drug designations accelerate development. This opportunity aligns with increased R&D investment in rare diseases, fostering innovation in precision medicine for JS
- For instance, in March 2025, a U.S.-based biotech initiated a Phase I trial for a CEP290-targeted gene therapy, reporting restored retinal function in JS mouse models, with human dosing planned for 2026
- Patient registries, like those supported by the Joubert Syndrome Foundation, are providing critical data for trial design. These efforts are spurring market growth by attracting investment and expanding therapeutic options for JS patients
- The gene therapy pipeline and clinical research growth present significant opportunities for Joubert Syndrome treatment, driving innovation and market expansion through targeted therapies
- Advances in gene therapy and collaborative research are creating transformative opportunities for Joubert Syndrome treatment, enhancing the market’s long-term potential through innovative solutions
Restraint/Challenge
“Limited Access and High Cost of Specialized Interventions"
- Despite improved recognition, access to multidisciplinary care for Joubert Syndrome remains limited in low-resource settings, particularly in LMICs, due to a lack of pediatric neurology expertise and advanced diagnostics like WES and MRI
- The high cost of supportive devices (e.g., mobility aids), genetic testing (>$5,000 per panel), and investigational therapies poses significant barriers. These challenges, compounded by low health literacy and stigma, hinder timely intervention, impacting market growth in underserved regions
- For instance, a 2023 Lancet study highlighted that only 10% of sub-Saharan African hospitals had access to MRI for MTS detection, delaying JS diagnoses by an average of 24 months
- Public-private partnerships aim to address affordability, but scalability remains a challenge, restricting market penetration
- Limited access and high costs of specialized interventions continue to restrain the Joubert Syndrome treatment market, necessitating innovative solutions to improve affordability and reach
- High costs and limited access to specialized care in low-resource settings pose significant challenges to the Joubert Syndrome treatment market, requiring targeted interventions to enhance equity and market growth
Joubert Syndrome Treatment Market Scope
The market is segmented on the basis of genes type, therapy type, drugs, route of administration, end users, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Genes Type |
|
|
By Therapy Type |
|
|
By Drugs |
|
|
By Route of Administration |
|
|
By End User |
|
|
By Distribution Channel
|
|
In 2025, the physical therapy is projected to dominate the market with a largest share in therapy type segment
In 2025, the physical therapy segment is expected to account for the highest growth rate and largest market share of 42.28%, driven by its essential role in enhancing neuromotor development and managing hypotonia, a core symptom of Joubert Syndrome. Physical therapy helps improve core strength, balance, posture, and motor coordination, enabling better participation in daily activities and reducing long-term musculoskeletal complications. Increasing adoption of early intervention programs, along with personalized rehabilitation protocols in clinical and homecare settings, is further reinforcing its widespread application.
The JBTS1 segment expected to account for the largest share during the forecast period in end user segment
In 2025, the JBTS1 segment is projected to hold a market share of 31.22%, owing to the gene’s high prevalence among confirmed Joubert Syndrome cases and its central role in clinical genetic diagnosis. JBTS1 mutations are one of the earliest and most widely studied genetic defects associated with the syndrome, facilitating their prioritization in first-line gene panels. The segment's growth is supported by increasing availability of next-generation sequencing (NGS) technologies, expanded newborn screening initiatives, and research into JBTS1’s function in primary cilia formation.
Joubert Syndrome Treatment Market Regional Analysis
“North America Holds the Largest Share in the Joubert Syndrome Treatment Market”
- North America dominates the Joubert Syndrome treatment market with a market share of approximately 41.62%, led by the United States. This dominance is driven by high clinical awareness, early diagnosis practices, and strong funding support for rare genetic disorders
- The U.S. leads the region with an estimated 32.81% share, supported by newborn screening programs, access to advanced genetic testing, and comprehensive multidisciplinary care models
- Government initiatives such as the Rare Disease Clinical Research Network (RDCRN) and NIH funding for rare neurodevelopmental syndromes ensure a robust ecosystem for diagnosis and long-term care
- The region benefits from established centers of excellence for rare disease management, integration of physical and occupational therapies, and access to emerging gene therapies in clinical trial settings
- The FDA’s orphan drug designations and fast-track pathways accelerate therapeutic development and approval for Joubert Syndrome-related treatments, promoting faster access to novel interventions
- Major players such as Pfizer, Biogen, Ionis, and PTC Therapeutics are actively operating in North America, driving both research innovation and commercialization of potential therapies
“Asia-Pacific is Projected to Register the Highest CAGR in the Joubert Syndrome Treatment Market”
- Asia-Pacific is projected to grow at the fastest pace and currently holds an estimated market share of 20.3%, fueled by expanding diagnostic capabilities and increased awareness of rare pediatric neurological disorders
- India and China are leading contributors, with rising investment in genomics, newborn screening expansion, and increasing collaborations between research institutes and global biotech firms
- Several governments across the region are launching rare disease policies and national registries, which are improving early identification, monitoring, and patient support systems.
- In countries like China, national genomics initiatives and AI-powered diagnostic labs are accelerating the detection of ciliopathies such as Joubert Syndrome
- India is witnessing a surge in public-private partnerships to scale up genetic testing accessibility and pediatric rehabilitation centers in metro and tier-2 cities
- Japan and South Korea are emerging leaders in adopting experimental gene therapies and advanced neurorehabilitation techniques due to strong R&D ecosystems and insurance-backed support for rare diseases
Joubert Syndrome Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Pfizer Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Novartis AG (Switzerland)
- Takeda Pharmaceutical Company Limited (Japan)
- Ipsen (France)
- Merck & Co., Inc. (U.S.)
- Biogen Inc. (U.S.)
- Sanofi (France)
- Ionis Pharmaceuticals, Inc. (U.S.)
- Vertex Pharmaceuticals Incorporated (U.S.)
- Regenxbio Inc. (U.S.)
- PTC Therapeutics, Inc. (U.S.)
- Orphazyme A/S (Denmark)
- Zogenix, Inc. (U.S.)
- Amicus Therapeutics, Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- AstraZeneca (U.K.)
- Amgen Inc. (U.S.)
- Alexion Pharmaceuticals, Inc. (U.S.)
- Gilead Sciences, Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Eli Lilly and Company (U.S.)
- Regeneron Pharmaceuticals, Inc. (U.S.)
Latest Developments in Global Joubert Syndrome Treatment Market
- In January 2025, Ionis Pharmaceuticals announced preclinical results for ION-CEP290-2.5Rx, an antisense oligonucleotide (ASO) therapy targeting CEP290 mutations associated with Joubert Syndrome.Ionis reported promising in vivo data demonstrating that ION-CEP290-2.5Rx significantly restored CEP290 protein expression and improved ciliary function in animal models. The therapy specifically targets the aberrant splicing caused by CEP290 mutations—a major contributor to Joubert pathology—offering a mutation-specific intervention. These findings support the potential of ASO platforms in treating ciliopathies like Joubert Syndrome and position Ionis to enter IND-stage development by late 2025, pending toxicology validation
- In October 2024, Regenxbio initiated IND-enabling studies for a gene therapy candidate aimed at TMEM67 mutations in Joubert patients, with Phase I trials expected to begin in early 2026.The candidate therapy leverages Regenxbio’s NAV® AAV9 vector platform, designed to deliver a functional copy of the TMEM67 gene directly to cerebellar and renal tissues affected in Joubert Syndrome. Preclinical data from animal models have shown successful CNS transduction and gene expression. This program marks one of the first gene therapies specifically addressing TMEM67-associated Joubert subtypes, which are linked to both neurological and renal involvement
- In August 2024, Roche began a collaborative pediatric neurological research program with U.S. universities to investigate molecular pathways underlying Joubert-related cerebellar hypoplasia.This multi-institutional initiative focuses on dissecting the signaling mechanisms disrupted in cerebellar development, particularly those regulated by ciliopathy-associated genes such as AHI1, CC2D2A, and TMEM67. The partnership includes advanced imaging, transcriptomic profiling, and iPSC-derived neuronal models to map disease pathways and identify viable molecular targets. Findings from this effort are expected to inform novel therapeutic approaches and neuroprotective strategies for early-onset Joubert Syndrome
- In March 2024, Amicus Therapeutics received Orphan Drug Designation (ODD) from the U.S. FDA for a novel substrate reduction therapy (SRT) targeting renal dysfunction in Joubert Syndrome.The investigational therapy aims to mitigate nephronophthisis—a common and progressive renal manifestation in Joubert patients—by reducing the accumulation of toxic metabolites that impair kidney function. Amicus’s candidate works through a small-molecule mechanism designed for chronic administration and has shown early promise in preclinical nephron models. The ODD status provides regulatory incentives such as tax credits, user fee waivers, and potential market exclusivity, accelerating the development pathway
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.