Global Lambert Eaton Myasthenic Syndrome Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
820.00 Million
USD
1,363.23 Million
2024
2032
USD
820.00 Million
USD
1,363.23 Million
2024
2032
| 2025 –2032 | |
| USD 820.00 Million | |
| USD 1,363.23 Million | |
|
|
|
|
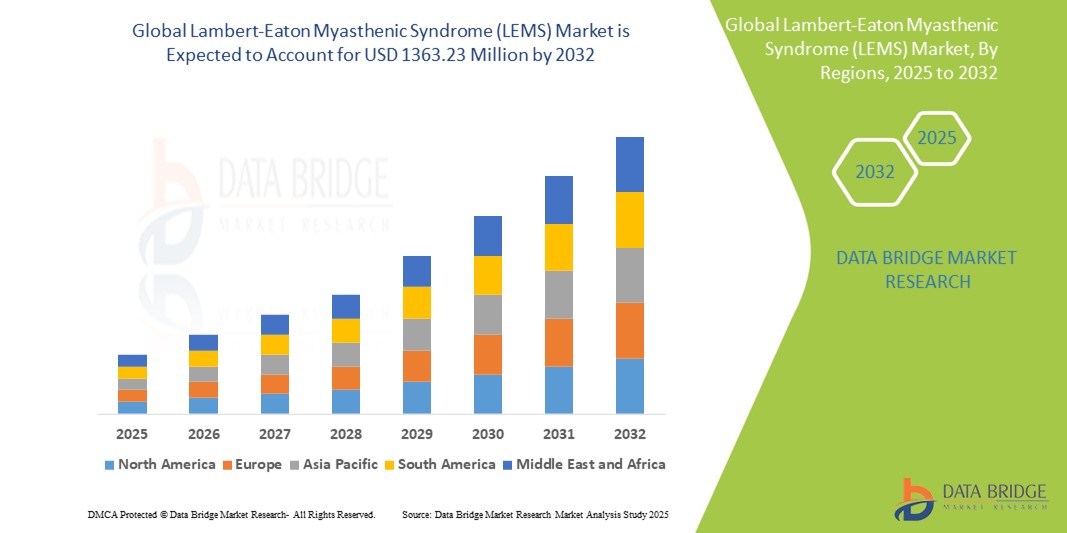
Lambert-Eaton Myasthenic Syndrome (LEMS) Market Size
- The Global Lambert-Eaton Myasthenic Syndrome (LEMS) Treatment market size was valued at USD 820 million in 2024 and is expected to reach USD 1,363.23 million by 2032, at a CAGR of 6.56% during the forecast period
- This growth is driven by increased diagnosis of rare neuromuscular disorders, regulatory incentives for orphan drugs, and rising awareness among neurologists and oncologists
Lambert-Eaton Myasthenic Syndrome (LEMS) Market Analysis
- LEMS is a rare autoimmune disorder characterized by muscle weakness of the limbs, often associated with underlying malignancies such as small-cell lung cancer. The condition is treated using symptomatic therapies like potassium channel blockers and immunomodulatory treatments such as corticosteroids and IVIG
- The market is growing due to advancements in targeted drug development, improved patient registries, and expanded access to immunotherapy in rare disease centers
- North America holds the largest market share due to faster diagnosis, high availability of orphan drugs, and advanced healthcare systems
- Asia-Pacific is witnessing rising diagnostic rates, with governments initiating rare disease coverage policies and funding registries to improve disease tracking and patient access
- The potassium channel blockers segment is projected to dominate the market with the largest share of 48.1%, due to their primary role in managing neuromuscular transmission symptoms and regulatory recognition as the standard of care in LEMS
Report Scope and Lambert-Eaton Myasthenic Syndrome (LEMS) Market Segmentation
|
Attributes |
Lambert-Eaton Myasthenic Syndrome (LEMS) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Lambert-Eaton Myasthenic Syndrome (LEMS) Market Trends
"Increased Focus on Rare Disease-Specific Therapeutic Development"
- The Lambert-Eaton Myasthenic Syndrome (LEMS) treatment market is undergoing a shift toward more targeted, disease-specific therapies.Drug development is increasingly centered around the autoimmune nature of LEMS, with a focus on modulating calcium and potassium ion channels involved in neuromuscular transmission
- Alongside precision-targeted molecules, companies are also investing in long-acting formulations to reduce the frequency of administration and improve patient adherence
- For instance, in 2024, Catalyst Pharmaceuticals reported significant progress in its Phase III trial for an extended-release version of amifampridine, which aims to maintain stable therapeutic levels with fewer doses per day
- These advancements represent a clear move toward personalized and convenient treatments for LEMS, with the potential to improve both clinical outcomes and quality of life for patients by minimizing the daily treatment burden.
Driver:
"Rise in Early Diagnosis and Expansion of Orphan Drug Approvals"
- Early and accurate diagnosis of LEMS is improving globally due to better access to antibody testing (anti-VGCC) and advanced neuromuscular diagnostic tools. These improvements are reinforced by regulatory frameworks that incentivize rare disease innovation
- Orphan drug designations provide developers with market exclusivity, tax credits, and accelerated regulatory pathways
- For instance, in 2023, the U.S. FDA granted Priority Review status to a novel potassium channel blocker for both adult and pediatric LEMS cases, reflecting the urgency and unmet need in this niche market
- These factors collectively foster a more supportive environment for innovation, accelerating the availability of novel therapies and enabling early treatment interventions in LEMS, which can significantly alter disease progression.
Opportunity
"Strategic Collaborations and Expanded Access Programs"
- To improve global access, pharmaceutical companies are increasingly entering into collaborations with academic institutions, rare disease foundations, and public health entities
- These partnerships are helping generate real-world data, support early access initiatives, and enable drug availability in underserved regions through compassionate use programs
- For Instance, in 2024, Jacobus Pharmaceuticals partnered with a European rare disease consortium to supply amifampridine free of cost to low-income patient populations under an expanded access scheme
- Such alliances enhance treatment equity, particularly in resource-limited settings, and help build the clinical evidence base for broader regulatory and reimbursement approvals.
Restraint/Challenge:
"High Treatment Costs and Limited Specialist Availability"
- Despite advancements, LEMS treatment remains costly, especially with IVIG and advanced neuromodulators that require hospital-based or supervised administration
- In low- and middle-income regions, diagnosis and treatment are further delayed by the scarcity of neurology specialists and lack of awareness among general physicians
- For instance, In 2023 report by Rare Disease Europe highlighted that over 60% of LEMS patients in low-income countries experienced diagnostic delays exceeding 18 months, leading to worsened prognosis and limited treatment response
- These barriers highlight the urgent need for cost-effective therapeutic options, improved diagnostic training, and broader distribution of neuromuscular expertise to ensure timely and equitable care delivery.
Lambert-Eaton Myasthenic Syndrome (LEMS) Market Scope
The market is segmented on the basis of type, drug class, route of administration, indication, distribution channel, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Drug Class |
|
|
By Route of Administration |
|
|
By Indication |
|
|
By End User |
|
|
By Distribution Channel
|
|
In 2025, the Potassium Channel Blockers is projected to dominate the market with a largest share in drug type segment
In 2025, potassium channel blockers are projected to lead the neuromuscular disorder drug market with a 48.1% share in the drug type segment. This dominance is attributed to their effectiveness in improving neuromuscular transmission symptoms, particularly in Lambert-Eaton Myasthenic Syndrome (LEMS), where they are recognized as the standard of care by regulatory bodies.
The Autoimmune LEMS is expected to account for the largest share during the forecast period in indication market
The autoimmune LEMS segment is forecasted to dominate the indication landscape with a 64.7% share in 2025. This growth is primarily driven by a rise in idiopathic autoimmune cases, especially in patients without underlying malignancies. Increased awareness campaigns and broader availability of antibody testing are enabling earlier and more accurate diagnosis. As a result, targeted treatment and management strategies for autoimmune LEMS are gaining traction, contributing to the segment’s expansion.
Lambert-Eaton Myasthenic Syndrome (LEMS) Market Regional Analysis
“North America Holds the Largest Share in the Lambert-Eaton Myasthenic Syndrome (LEMS) Market”
- North America accounts for the highest share of 45.21% due to rapid uptake of orphan drugs, availability of dedicated rare disease centers, and favorable reimbursement policies
- The U.S. leads with 65.12% market share with strong regulatory support and consistent trial activity in neuromuscular disorders
- The region boasts a network of dedicated neuromuscular and rare disease centers, such as the NIH Rare Diseases Clinical Research Network in the U.S., providing multidisciplinary care, clinical trial access, and advanced diagnostic support
- Reimbursement frameworks, especially in the U.S., support access to high-cost treatments such as gene therapies and biologics through public and private insurance schemes, reducing financial barriers for patients
- The U.S. leads globally in drug approvals and clinical research for neuromuscular disorders, thanks to fast-track and breakthrough therapy designations by the FDA, as well as significant funding for research and development from institutions like the NIH and pharmaceutical companies
“Asia-Pacific is Projected to Register the Highest CAGR in the Lambert-Eaton Myasthenic Syndrome (LEMS) Market”
- Asia-Pacific is anticipated to grow at the fastest rate and 15.32% market share owing to rising rare disease diagnosis, dedicated national funding, and international collaborations
- Countries like India and China are advancing early diagnosis programs for neuromuscular disorders through newborn and pediatric screening drives
- South Korea and Japan have implemented national rare disease registries and strong reimbursement pathways for IVIG and immunosuppressive therapies
- In additiona, regional pharmaceutical firms are expanding access through public-private partnerships and biosimilar development, making key LEMS treatments more affordable and accessible across emerging markets
Lambert-Eaton Myasthenic Syndrome (LEMS) Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Catalyst Pharmaceuticals Inc. (U.S.)
- Jacobus Pharmaceutical (U.S.)
- Grifols S.A. (Spain)
- CSL Behring (U.S.)
- Hansa Biopharma AB (Sweden)
- Takeda Pharmaceutical Company Limited (Japan)
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- BioMarin Pharmaceutical Inc. (U.S.)
- Alexion Pharmaceuticals Inc. (U.S.)
- argenx SE (Netherlands)
- Immunovant Inc. (U.S.)
- Ra Pharmaceuticals Inc. (U.S.)
- Bausch Health Companies Inc. (Canada)
- Prestige Biopharma Limited (Singapore)
- Zydus Cadila (India)
- Alvogen (Iceland)
- Apnar Pharma (U.S.)
- Novitium Pharma (U.S.)
- Amneal Pharmaceuticals LLC (U.S.)
Latest Developments in Global Lambert-Eaton Myasthenic Syndrome (LEMS) Treatment Market
- In March 2025, Catalyst Pharmaceuticals submitted a New Drug Application to the FDA for an extended-release amifampridine formulation.Catalyst Pharmaceuticals advanced its drug pipeline by filing a New Drug Application (NDA) with the U.S. FDA for an extended-release version of amifampridine. This formulation is designed to maintain stable therapeutic levels over a longer period, potentially reducing dosing frequency and improving patient adherence. If approved, it would offer a more convenient alternative for LEMS patients currently reliant on multiple daily doses
- In December 2024, Grifols announced the expansion of its IVIG production facility to meet rising global demand for rare disease treatments.Grifols responded to growing demand for intravenous immunoglobulin (IVIG) therapies, commonly used in the treatment of neuromuscular autoimmune disorders like LEMS, by expanding its manufacturing capacity. The facility upgrade aims to enhance global supply resilience and reduce shortages, particularly as demand for rare disease therapies increases across developed and emerging markets
- In August 2024, Hansa Biopharma began clinical trials for its anti-IgG enzyme therapy in antibody-positive LEMS patients.Hansa Biopharma initiated clinical trials of its investigational anti-IgG enzyme therapy, which is designed to break down pathogenic antibodies in patients with antibody-positive LEMS. This targeted approach seeks to offer a novel immunomodulatory treatment pathway, potentially benefiting patients who do not respond adequately to current potassium channel blockers or immunoglobulin therapies
- In May 2024, Takeda initiated a multicenter study on its investigational immunotherapy for refractory neuromuscular autoimmune syndromes.Takeda Pharmaceuticals launched a multicenter clinical study evaluating the efficacy of a novel immunotherapy for patients with refractory neuromuscular autoimmune syndromes, including LEMS. The study aims to explore alternative pathways for disease control in patients who have failed to respond to existing treatments, reinforcing the company’s focus on advancing care in rare and underserved indications
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.