Global Legionella Testing Market
Market Size in USD Billion
CAGR :
% 
 USD
368.50 Billion
USD
711.40 Billion
2025
2033
USD
368.50 Billion
USD
711.40 Billion
2025
2033
| 2026 –2033 | |
| USD 368.50 Billion | |
| USD 711.40 Billion | |
|
|
|
|
Legionella Testing Market Size
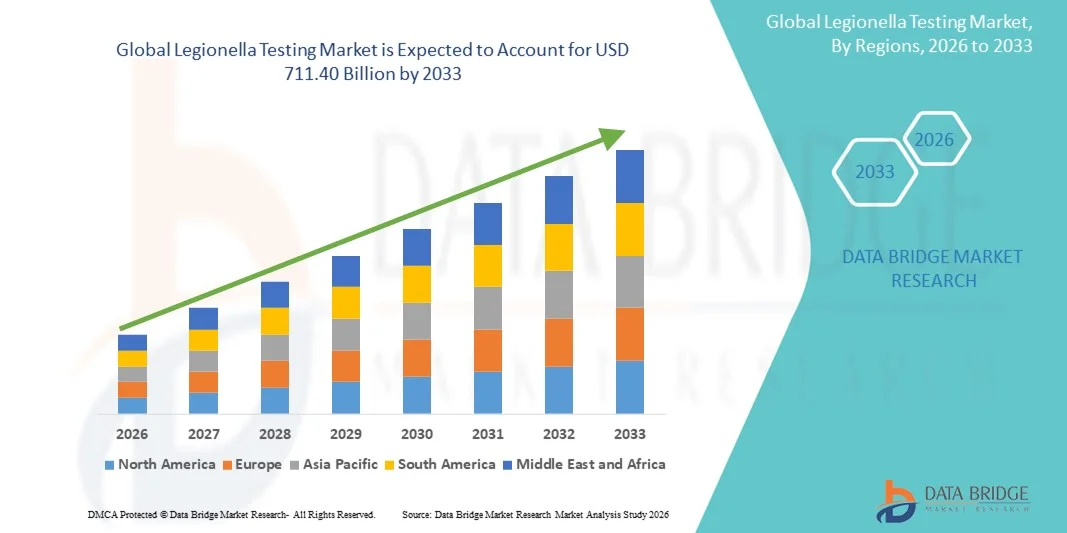
- The global legionella testing market size was valued at USD 368.50 billion in 2025 and is expected to reach USD 711.40 billion by 2033, at a CAGR of 8.57% during the forecast period
- The market growth is largely fueled by increasing awareness of waterborne diseases, stringent government regulations, and technological advancements in rapid and accurate Legionella detection methods, leading to greater adoption in commercial, industrial, and residential sectors
- Furthermore, rising concerns regarding public health and water safety, coupled with the demand for preventive monitoring and risk management solutions, are accelerating the uptake of Legionella Testing solutions, thereby significantly boosting the industry's growth
Legionella Testing Market Analysis
- Legionella Testing solutions, including rapid detection kits, culture-based methods, and PCR-based assays, are increasingly vital components of water safety management and public health monitoring in both residential, commercial, and industrial settings due to their ability to ensure early detection, prevent outbreaks, and maintain compliance with regulatory standards
- The escalating demand for legionella testing is primarily fueled by growing awareness of waterborne diseases, stringent government regulations, rising concerns regarding public health, and a preference for accurate, reliable, and timely monitoring solutions that can minimize risk and ensure safe water systems
- North America dominated the legionella testing market with the largest revenue share of 42% in 2025, characterized by advanced laboratory infrastructure, high adoption of routine water testing protocols, and the presence of key industry players. The U.S. contributed the majority of the regional share due to proactive public health policies, strong regulatory enforcement, and widespread use of automated and culture-based testing methods
- Asia-Pacific is expected to be the fastest growing region in the Legionella Testing market during the forecast period, registering a CAGR from 2026 to 2033, driven by rapid urbanization, increasing construction of commercial and healthcare facilities, government initiatives promoting water safety, and rising awareness of Legionnaires’ disease in countries such as China, India, and Japan
- The Environmental Testing Methods segment held the largest market revenue share of 49.2% in 2025, owing to widespread implementation in water management programs for hotels, hospitals, and industrial plants
Report Scope and Legionella Testing Market Segmentation
|
Attributes |
Legionella Testing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
• Roche Diagnostics (Switzerland) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Legionella Testing Market Trends
Rising Focus on Water Safety and Regulatory Compliance
- A key and accelerating trend in the global legionella testing market is the growing emphasis on water safety and regulatory compliance across residential, commercial, and industrial facilities. Governments and health agencies are increasingly mandating routine testing of water systems to prevent outbreaks of Legionnaires’ disease
- For instance, in 2023, the U.S. Centers for Disease Control and Prevention (CDC) updated its guidelines for water system monitoring in large buildings, encouraging more frequent Legionella testing and risk assessments
- The adoption of advanced testing kits and rapid detection methods is reshaping expectations for water quality monitoring. Facilities are investing in more precise and faster testing solutions to minimize health risks and regulatory penalties
- Facilities such as hospitals, hotels, and industrial complexes are now integrating routine Legionella monitoring into their standard operating procedures, highlighting a shift toward proactive risk management
- The increasing prevalence of aging plumbing systems in developed markets and the expansion of large-scale water infrastructure in emerging economies further drive adoption of reliable testing solutions
- These trends are fundamentally reshaping the Legionella Testing market, creating sustained demand for both consumables and analytical systems capable of rapid and accurate detection
- Companies such as IDEXX Laboratories and Bio-Rad are expanding their product portfolios with specialized Legionella testing kits to meet rising regulatory and health safety requirements
Legionella Testing Market Dynamics
Driver
Growing Need Due to Health Concerns and Industrial Safety Regulations
- The increasing prevalence of Legionnaires’ disease outbreaks and the associated public health risks is a significant driver for market growth. Awareness of the severe consequences of Legionella contamination is prompting organizations to adopt routine testing protocols
- For instance, in May 2024, Bio-Rad Laboratories launched a new rapid Legionella PCR assay designed for hospitals and water treatment facilities, enabling faster identification and mitigation of contamination risks
- Facilities are now prioritizing preventive testing measures, especially in high-risk environments such as hospitals, nursing homes, hotels, and large commercial buildings
- Government regulations, including OSHA standards and EU directives on water hygiene, mandate regular monitoring of water systems, further accelerating the market adoption of testing solutions
- The convenience of rapid on-site testing and automated monitoring systems also drives demand, allowing organizations to reduce downtime and respond quickly to contamination events
- Increased awareness campaigns and training initiatives are educating facility managers and public health officials on the importance of early detection, leading to higher testing frequency and broader adoption
- Overall, the rising focus on health and industrial safety creates a sustained, growing market for Legionella testing products and services
Restraint/Challenge
High Testing Costs and Technical Complexity
- Challenges associated with the relatively high cost of advanced Legionella testing kits and laboratory equipment can limit widespread adoption, particularly among small businesses and developing regions
- For instance, a municipal water facility in India reported that the cost of adopting a real-time PCR-based Legionella testing system was nearly 3–4 times higher than conventional culture-based methods, discouraging small-scale implementation
- Furthermore, technical complexity and the need for skilled laboratory personnel to conduct and interpret certain testing protocols may pose barriers, especially in regions with limited access to trained microbiologists
- The reliance on multiple reagents, calibration standards, and precise handling procedures increases operational costs and can impact testing consistency if staff are inadequately trained
- Despite cost reductions and simplifications in recent rapid detection technologies, budget constraints and infrastructural limitations continue to hinder adoption in some markets
- Addressing these challenges requires industry players to offer more cost-effective solutions, modular testing kits, and educational support for facility operators, ensuring reliability and regulatory compliance without imposing prohibitive costs
- Overcoming the cost and complexity barriers will be critical for enabling broader, sustained adoption of Legionella testing solutions across diverse global markets
Legionella Testing Market Scope
The market is segmented on the basis of test type, testing method, application, and end-user.
- By Test Type
On the basis of test type, the Legionella Testing market is segmented into Culture Methods, UAT, Serology, DFA Test, Nucleic Acid-Based Detection, and PCR. The Culture Methods segment dominated the largest market revenue share of 41.5% in 2025, owing to its status as the gold standard for Legionella detection and widespread acceptance across clinical and environmental laboratories. Culture methods provide reliable confirmation of viable bacteria in water systems, which is critical for compliance with regulatory standards. Hospitals, large hotels, and industrial facilities rely heavily on culture methods due to their accuracy, reproducibility, and ability to quantify bacterial load. In addition, the simplicity of routine culture kits and the familiarity among laboratory staff support continued adoption. Established providers like Bio-Rad and IDEXX Laboratories maintain strong distribution of culture-based kits globally. The segment benefits from standardized protocols recommended by WHO and CDC, reinforcing trust in results. Culture methods also allow long-term sample storage and retrospective analysis, valuable for epidemiological studies. Despite being relatively time-consuming, their reliability ensures they remain dominant across developed regions such as North America and Europe. Investments in water safety programs and building compliance initiatives further support market growth.
The PCR-based detection segment is expected to witness the fastest CAGR of 22.3% from 2026 to 2033, driven by the increasing demand for rapid, sensitive, and specific detection solutions in clinical diagnostics and environmental monitoring. PCR enables detection of low bacterial concentrations within hours, unlike traditional culture methods that take several days. Fast turnaround is particularly critical during outbreak investigations in hospitals and hotels. Advancements in multiplex PCR and real-time PCR assays have improved diagnostic accuracy and reduced contamination risks. Portable and automated PCR devices are increasingly adopted for on-site testing in industrial water systems. Regulatory approvals in multiple countries foster adoption in North America, Europe, and Asia-Pacific. Awareness of Legionnaires’ disease and technological innovations in nucleic acid-based testing further accelerate market expansion. The rising need for early outbreak detection in water systems and hospitals enhances the segment’s growth trajectory.
- By Testing Method
On the basis of testing method, the Legionella Testing market is segmented into Water Testing and IVD Testing. The Water Testing segment accounted for the largest market revenue share of 47.8% in 2025, driven by mandatory water system monitoring in hospitals, hotels, and commercial buildings. Organizations prioritize water testing due to regulations requiring proof of safety in potable water, cooling towers, and plumbing systems. Standardized testing protocols and routine maintenance schedules support consistent adoption. Water testing provides actionable results for preventive measures, helping facility managers mitigate contamination risks efficiently. Countries like the U.S., Germany, and Japan have stringent guidelines for Legionella testing in water, bolstering the market. Providers offer integrated water testing kits combining sample collection, culture, and rapid detection methods, enhancing convenience and reliability. Rising awareness of Legionella contamination in commercial buildings fuels adoption. Environmental regulations and government compliance programs also drive market demand.
The IVD Testing segment is expected to register the fastest CAGR of 19.8% from 2026 to 2033, propelled by the rising incidence of hospital-acquired Legionnaires’ disease and the need for rapid patient diagnosis. IVD solutions, including urinary antigen tests and serology kits, allow quick clinical diagnosis, enabling timely treatment and reducing mortality. Hospitals and diagnostic laboratories are increasingly integrating IVD testing into routine screening programs, particularly in immunocompromised patients. Automated ELISA and lateral flow devices further support adoption. Government health initiatives and rising awareness of early diagnosis drive segment growth.
- By Application
On the basis of application, the market is segmented into Clinical Testing Methods and Environmental Testing Methods. The Environmental Testing Methods segment held the largest market revenue share of 49.2% in 2025, owing to widespread implementation in water management programs for hotels, hospitals, and industrial plants. Environmental testing is crucial for outbreak prevention and compliance with local and international guidelines. The segment’s dominance is driven by mandatory building safety codes, rising litigation risks, and the expansion of commercial infrastructure in North America and Europe. Environmental testing provides preventive insights and actionable data to mitigate contamination in water systems. Providers increasingly offer bundled services including sampling, culture, and rapid testing. Countries with strict water safety regulations encourage continuous adoption. The segment also benefits from technological advancements in automated water testing equipment.
The Clinical Testing Methods segment is projected to witness the fastest CAGR of 20.5% from 2026 to 2033, driven by increasing awareness of early diagnosis and treatment of Legionnaires’ disease in hospitals and clinics. Rapid clinical assays, PCR-based detection, and point-of-care testing facilitate timely diagnosis and improve patient outcomes. Rising prevalence of nosocomial infections in aging populations across Europe, North America, and Asia-Pacific accelerates adoption. Hospitals are implementing routine Legionella screening protocols for high-risk patients. Advanced diagnostics improve patient care while reducing outbreak risks. Technological innovation, government regulations, and awareness campaigns boost segment growth.
- By End-User
On the basis of end-user, the Legionella Testing market is segmented into Hospitals & Clinics and Diagnostic Laboratories. The Diagnostic Laboratories segment dominated the largest revenue share of 52% in 2025, supported by high demand for outsourced Legionella testing services from commercial buildings, hotels, and municipal water systems. Laboratories offer specialized testing capabilities, including culture, PCR, and multiplex assays, providing accurate results to both healthcare and environmental clients. The segment benefits from established laboratory networks, accreditation programs, and partnerships with commercial clients. Large-scale laboratories in North America and Europe dominate this segment due to advanced capabilities.
The Hospitals & Clinics segment is expected to record the fastest CAGR of 21% from 2026 to 2033, fueled by increased testing frequency for immunocompromised and elderly patients. Hospitals are adopting rapid diagnostics and point-of-care solutions to reduce hospital-acquired infection rates. Rising awareness, technological advances, and health initiatives drive segment growth. The expansion of hospital infrastructure in emerging markets also contributes to higher adoption rates. In addition, partnerships with diagnostic service providers and integration of automated testing platforms are enhancing testing efficiency and accuracy. Growing investments in healthcare IT and laboratory information management systems further support the segment’s robust growth trajectory.
Legionella Testing Market Regional Analysis
- North America dominated the legionella testing market with the largest revenue share of 42% in 2025, characterized by advanced laboratory infrastructure, high adoption of routine water testing protocols, and the presence of key industry players
- The market contributed the majority of the regional share due to proactive public health policies, strong regulatory enforcement, and widespread use of automated and culture-based testing methods
- The region’s market growth is further supported by increasing awareness about Legionnaires’ disease, government-led monitoring programs, and investments in modern water safety management systems
U.S. Legionella Testing Market Insight
The U.S. legionella testing market captured the largest revenue share in 2025 within North America, driven by stringent federal and state regulations, routine inspections in healthcare and hospitality sectors, and rising demand for rapid detection technologies. The adoption of PCR-based and culture-based testing methods, coupled with growing awareness of Legionella prevention, significantly contributes to market expansion.
Europe Legionella Testing Market Insight
The Europe legionella testing market is projected to expand at a substantial CAGR throughout the forecast period, primarily fueled by strict regulatory standards, mandatory water testing in public facilities, and rising investments in water safety infrastructure. Countries like Germany, France, and Italy are witnessing increased adoption of advanced detection technologies and preventive testing programs.
U.K. Legionella Testing Market Insight
The U.K. legionella testing market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by heightened public awareness, regulatory compliance requirements in healthcare and hospitality sectors, and adoption of automated testing systems for faster and more reliable results.
Germany Legionella Testing Market Insight
The Germany legionella testing market is expected to expand at a considerable CAGR during the forecast period, driven by robust water safety regulations, a high concentration of healthcare and industrial facilities, and increasing adoption of rapid diagnostic techniques for Legionella detection.
Asia-Pacific Legionella Testing Market Insight
The Asia-Pacific legionella testing market is poised to grow at the fastest CAGR during the forecast period of 2026 to 2033, driven by rapid urbanization, increasing construction of commercial and healthcare facilities, government initiatives promoting water safety, and rising awareness of Legionnaires’ disease in countries such as China, India, and Japan.
Japan Legionella Testing Market Insight
The Japan legionella testing market is gaining momentum due to growing awareness of Legionnaires’ disease, stringent government water safety regulations, and increasing adoption of automated and culture-based testing methods across commercial, healthcare, and municipal water systems.
China Legionella Testing Market Insight
The China legionella testing market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rapid urbanization, expansion of commercial and healthcare infrastructure, rising public health awareness, and increased regulatory enforcement for water safety. The availability of cost-effective and advanced detection solutions from domestic and international suppliers is further propelling market growth.
Legionella Testing Market Share
The Legionella Testing industry is primarily led by well-established companies, including:
• Roche Diagnostics (Switzerland)
• Bio-Rad Laboratories (U.S.)
• Lonza Group (Switzerland)
• Merck KGaA (Germany)
• Charles River Laboratories (U.S.)
• Siemens Healthineers (Germany)
• PerkinElmer (U.S.)
• Biomerieux (France)
• Magle Life Sciences (Denmark)
• EnviroLogix (U.S.)
• ATCC (U.S.)
• Eurofins Scientific (Luxembourg)
• MDL (U.S.)
• Neogen Corporation (U.S.)
• Promega Corporation (U.S.)
• TCS Biosciences (U.K.)
• Hach Company (U.S.)
Latest Developments in Global Legionella Testing Market
- In June 2021, Phigenics launched a new diagnostic method, Next‑Day LegiPlex PCR, designed to detect Legionella serogroups and species in building water systems — enabling quicker detection compared with traditional culture-based methods
- In December 2021, Pace Analytical Services acquired Special Pathogens Laboratory — a leading lab for Legionella detection, remediation, and prevention — to strengthen its specialty‑contaminant testing and regulatory‑compliance services
- In September 2024, LuminUltra Technologies acquired the Legionella‑testing assets of Genomadix Inc., including a portable qPCR platform (the “Cube™”) capable of detecting Legionella in under an hour — expanding rapid, on‑site testing capabilities
- In September 2024, Pace Analytical Services also established a “National Center of Excellence for Legionella Testing and Consulting” in Pittsburgh, U.S., aiming to offer specialized testing, outbreak response and consulting services — indicating rising demand and institutional commitment to Legionella monitoring
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.