Global Localized Therapies In Alopecia Areata Market
Market Size in USD Billion
CAGR :
% 
 USD
2.04 Billion
USD
4.15 Billion
2024
2032
USD
2.04 Billion
USD
4.15 Billion
2024
2032
| 2025 –2032 | |
| USD 2.04 Billion | |
| USD 4.15 Billion | |
|
|
|
Localized Therapies in Alopecia Areata Market Analysis
The localized therapies in the Alopecia Areata market have shown significant growth due to their ability to directly target affected areas while minimizing systemic side effects. Key treatments include topical corticosteroids, topical immunotherapy (such as Diphenylcyclopropenone), and minoxidil, which are commonly used to stimulate hair regrowth and modulate immune responses locally.
Among these, topical corticosteroids are widely prescribed for mild to moderate cases due to their effectiveness in reducing inflammation and suppressing immune responses in the scalp area. Topical immunotherapy is gaining traction as an innovative approach, particularly for more severe forms, by triggering a controlled allergic reaction to promote hair regrowth. In addition, minoxidil, a well-established topical treatment, remains a preferred choice for its ability to promote hair follicle stimulation.
The growing preference for localized therapies can be attributed to their non-invasive nature, convenience, and cost-effectiveness compared to systemic treatments. Furthermore, localized treatments offer fewer side effects and a targeted approach, making them an attractive option for both patients and clinicians. The market continues to expand as new formulations and combination therapies emerge, aiming to enhance efficacy and patient satisfaction. This segment is expected to see robust growth with the increasing adoption of non-pharmacological and less invasive treatment methods.
Localized Therapies in Alopecia Areata Market Size
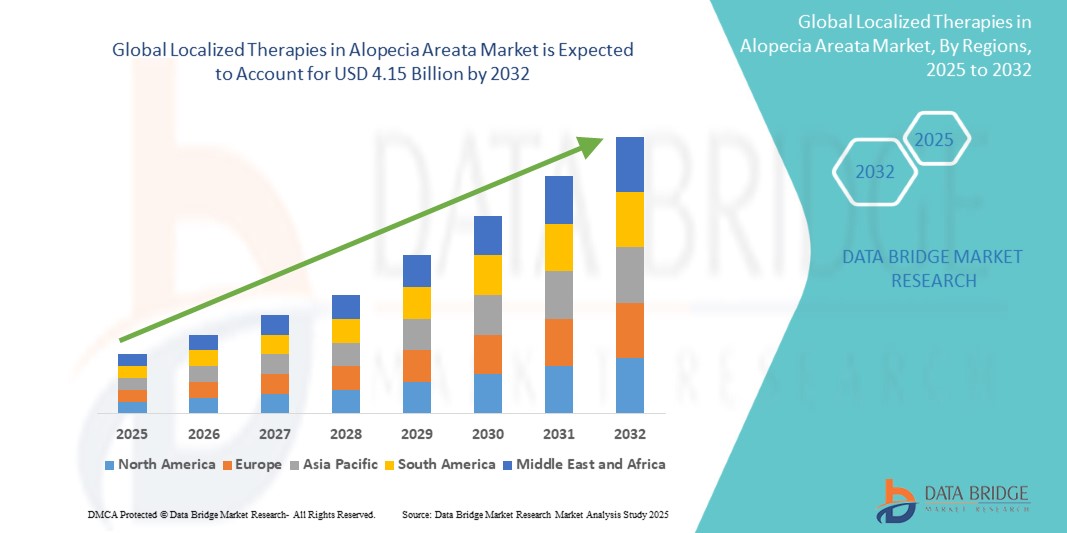
The global localized therapies in alopecia areata market size was valued at USD 2.04 billion in 2024 and is projected to reach USD 4.15 billion by 2032, with a CAGR of 9.27% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Localized Therapies in Alopecia Areata Market Trends
“Increasing Adoption of Topical Immunotherapy”
One prominent trend in the localized therapies for alopecia areata market is the increasing adoption of topical immunotherapy. This treatment, which includes agents such as Diphenylcyclopropenone (DPCP) and Squaric Acid Dibutyl Ester (SADBE), is gaining popularity due to its ability to stimulate hair regrowth by provoking a controlled allergic reaction on the scalp. As an alternative to traditional corticosteroid treatments, topical immunotherapy is especially effective for patients with more severe or resistant forms of alopecia areata, such as patchy or extensive hair loss.
This trend is driven by its non-invasive nature and relatively favorable safety profile compared to systemic therapies. In addition, patients and healthcare providers are increasingly seeking personalized, less harmful options that offer targeted action and fewer side effects. As more clinical evidence emerges supporting its efficacy, topical immunotherapy is expected to become a standard therapeutic option in the management of alopecia areata.
Report Scope and Localized Therapies in Alopecia Areata Market Segmentation
|
Attributes |
Localized Therapies in Alopecia Areata Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
Amgen Inc. (U.S.), AbbVie Inc. (U.S.), Aclaris Therapeutics, Inc. (U.S.), Bayer AG (Germany), Boehringer Ingelheim International GmbH (Germany), Equillium Bio (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), HaploX (Hong Kong), Incyte (U.S.), Lilly (U.S.), Novartis AG (Switzerland), Q32 Bio Inc. (U.S.), Pfizer Inc. (U.S.), Regeneron Pharmaceuticals Inc. (U.S.), Sun Pharmaceutical Industries Ltd. (India), Sanofi (France) and Viatris Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Localized Therapies in Alopecia Areata Market Definition
Localized therapies in alopecia areata refer to treatments that are directly applied or targeted to the affected areas of the scalp or skin to treat hair loss caused by the condition. These therapies aim to stimulate hair regrowth, reduce inflammation, or modulate the immune system locally, without affecting the entire body. Common localized treatments include topical corticosteroids, minoxidil, topical immunotherapy (such as Diphenylcyclopropenone), and topical immunomodulators (such as tacrolimus). These treatments are preferred for their non-invasive nature, ability to focus on specific regions, and fewer systemic side effects compared to oral or injectable treatments. Localized therapies are often used for mild to moderate cases or as part of a broader treatment strategy for more severe forms of alopecia areata.
Localized Therapies in Alopecia Areata Market Dynamics
Drivers
- Rising Demand for Non-Invasive Treatments
One of the key drivers of localized therapies in the Alopecia Areata market is the growing preference for non-invasive treatments. Patients are increasingly seeking solutions that avoid the risks and discomfort associated with oral medications and injections. Topical therapies, such as minoxidil and topical corticosteroids, offer a convenient, self-administered, and less painful alternative for managing hair loss. In addition, topical immunotherapy has emerged as a highly effective treatment for more severe cases, where other therapies may not have worked. For instance, treatments such as Diphenylcyclopropenone (DPCP) provoke an allergic reaction that promotes hair regrowth, offering a targeted approach without the need for systemic medication. This preference for non-invasive options enhances patient compliance and satisfaction, driving the market's growth. As patients demand more affordable and safer solutions, the demand for localized therapies is expected to increase, fueling market expansion.
- Advancements in Topical Drug Development
The continued advancement in topical drug development is another major driver in the localized therapies market for Alopecia Areata. Researchers are focused on creating more effective topical formulations that enhance the efficacy and safety of treatments. The development of JAK inhibitors such as Ruxolitinib in topical forms provides promising results for stimulating hair regrowth in patients with moderate to severe alopecia areata. These innovations are expected to improve outcomes, particularly in patients who may not respond well to conventional treatments such as corticosteroids. For instance, Topical JAK inhibitors have shown the potential to block immune-related pathways that cause hair follicle damage, offering a targeted and effective solution. The introduction of these advanced drugs leads to higher patient satisfaction and compliance, ultimately driving growth in the market. As pharmaceutical companies invest more in research and development, new and more efficient localized therapies will continue to emerge, further boosting market demand.
Opportunities
- Expansion of Personalized Medicine
One significant opportunity in the Localized Therapies for Alopecia Areata market lies in the growing trend of personalized medicine. Tailoring treatments based on a patient’s genetic profile, disease severity, and immune system response can lead to more effective and targeted therapies. For instance, the development of JAK inhibitors in topical formulations offers a more precise treatment approach for individuals who may not respond well to standard therapies such as corticosteroids. Personalized treatments ensure better outcomes, fewer side effects, and enhanced patient satisfaction. This opportunity is especially relevant as more advanced diagnostic tools are available to identify genetic markers that predict treatment efficacy. By offering treatments that are more closely aligned with each patient's unique needs, the market can expect improved compliance, quicker results, and higher market demand. Personalized localized therapies could thus become a key driver of market growth, meeting the growing patient demand for customized care options.
- Growth in Over-the-Counter (OTC) Products
Another promising opportunity in the localized therapies market for Alopecia Areata is the growth in Over-the-Counter (OTC) products. The increasing availability of minoxidil and other topical treatments without a prescription has made hair regrowth solutions more accessible to a wider audience. OTC therapies allow patients to manage their condition privately and cost-effectively, which appeals to those with mild cases of alopecia areata. An instance is the widespread use of minoxidil in topical form, which has been proven to stimulate hair growth in affected areas. The growth of online pharmacies and retail distribution channels further supports the expansion of OTC products. This opportunity not only drives market growth by reaching a broader demographic but also promotes patient self-care and early intervention. As consumer awareness increases, OTC localized therapies will likely see accelerated adoption, contributing to overall market expansion.
Restraints/Challenges
- Regulatory Hurdles and Approval Delays
A significant restraint in the market for localized therapies in Alopecia Areata is the lengthy and complex regulatory approval process. Developing and obtaining regulatory approval for new treatments involves extensive clinical trials, which can be time-consuming and costly. Regulatory bodies, such as the FDA, require substantial evidence of safety and efficacy before granting approval. For instance, while therapies such as JAK inhibitors show promise, their approval process has faced delays due to concerns over long-term safety. These regulatory hurdles often prevent quicker market entry of new localized treatments and limit the availability of innovative solutions for patients. The slow pace of approval can also stifle investment in research and development, further slowing market growth. As a result, the uncertainty in regulatory timelines and the burden of compliance can significantly hinder the expansion of the localized therapies market in Alopecia Areata.
- Limited Efficacy and Side Effects
Localized therapies for Alopecia Areata face challenges related to their efficacy and potential side effects. While treatments such as corticosteroids may provide temporary relief, their long-term effectiveness in sustaining hair regrowth remains uncertain for some patients. Furthermore, these therapies often come with adverse effects, such as skin thinning, irritation, or systemic side effects, especially when used over prolonged periods. For instance, immunotherapy using diphencyprone (DPCP) can lead to allergic reactions or dermatitis in some patients. These challenges make it difficult to maintain consistent treatment compliance, which could delay market adoption. In addition, the risk of side effects may lead patients to discontinue treatment, thereby limiting the overall growth of the localized therapies market. Despite their effectiveness for certain individuals, these factors represent significant barriers to widespread use and market expansion.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Localized Therapies in Alopecia Areata Market Scope
The market is segmented on the basis of therapy type and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Therapy Type
- Corticosteroids
- Topical Immunotherapy
- Anthraline
- Retinoid
- Prostaglandin Analogue
- Vitamin D Analog
- Intralesional corticosteroids
- Platelet-rich plasma
- Stem cells
- Minoxidil
- Tacrolimus
- Photo chemotherapy
- Superficial cryotherapy
- Others
Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
Localized Therapies in Alopecia Areata Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, therapy type, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the Localized Therapies in the Alopecia Areata market. This is primarily due to the high prevalence of Alopecia Areata in the region, coupled with advanced healthcare infrastructure, robust research and development (R&D) activities, and the presence of key pharmaceutical companies. In North America, particularly the United States, patients have access to innovative localized therapies such as JAK inhibitors and corticosteroid-based treatments, which are often covered by health insurance plans, making them more accessible. Moreover, ongoing clinical trials and significant investment in the development of new treatments contribute to market growth in this region.
Asia Pacific is expected to exhibit the highest growth rate in the Localized Therapies in the Alopecia Areata market. The region is experiencing rapid advancements in healthcare infrastructure, increasing awareness of autoimmune conditions, and growing adoption of innovative treatments. Countries such as Japan, China, and India have a high prevalence of Alopecia Areata, and with rising disposable incomes, there is a greater demand for advanced therapies.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Localized Therapies in Alopecia Areata Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Localized Therapies in Alopecia Areata Market Leaders Operating in the Market Are:
- Amgen Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Aclaris Therapeutics, Inc. (U.S.)
- Bayer AG (Germany)
- Boehringer Ingelheim International GmbH (Germany)
- Equillium Bio (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- HaploX (Hong Kong)
- Incyte (U.S.)
- Lilly (U.S.)
- Novartis AG (Switzerland)
- Q32 Bio Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Regeneron Pharmaceuticals Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Sanofi (France)
- Viatris Inc. (U.S.)
Latest Developments in Localized Therapies in Alopecia Areata Market
- In July 2024, Sun Pharmaceutical Industries Limited announced that the U.S. Food and Drug Administration (FDA) had approved LEQSELVI (deuruxolitinib) 8 mg tablets for the treatment of adults with severe alopecia areata. LEQSELVI, a JAK inhibitor, targets the root cause of alopecia areata and has been clinically proven to provide statistically significant efficacy.
- In June 2024, according to a published report in the The Harvard Gazette, Harvard researchers have developed a groundbreaking treatment to reverse hair loss caused by alopecia areata, an autoimmune condition. The team created a microneedle patch that promotes the regrowth of hair by enhancing immune cell recruitment to hair follicles. The patch showed promising results in reversing hair loss in mice and demonstrated excellent stability, offering hope for future clinical applications in humans. This discovery could revolutionize treatment for alopecia areata.
- In June 2024, Equillium Inc. announced positive topline results from its Phase 2, single-dose, proof-of-concept (PoC) study of EQ101 in adult patients with moderate to very-severe alopecia areata (AA), an autoimmune condition where immune cells attack hair follicles, leading to hair loss.
- In June 2023, Pfizer Inc. announced that the U.S. Food and Drug Administration (FDA) had granted approval for LITFULO (ritlecitinib), a once-daily oral treatment for individuals aged 12 and older with severe alopecia areata. The recommended dosage for LITFULO is 50 mg. This marks it as the first and only FDA-approved treatment for adolescents (12+) suffering from severe alopecia areata.
- In June 2022, Eli Lilly and Company and Incyte announced that the U.S. Food and Drug Administration (FDA) had approved OLUMIANT (baricitinib), a once-daily oral treatment, as the first systemic therapy for adults with severe alopecia areata (AA). The medication is available in 4-mg, 2-mg, and 1-mg tablets, with a recommended starting dose of 2 mg per day, which can be increased to 4 mg per day if the treatment response is insufficient.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.