Global Lysosomal Acid Lipase Deficiency Lal D Market
Market Size in USD Billion
CAGR :
% 
 USD
740.52 Billion
USD
1,587.37 Billion
2024
2032
USD
740.52 Billion
USD
1,587.37 Billion
2024
2032
| 2025 –2032 | |
| USD 740.52 Billion | |
| USD 1,587.37 Billion | |
|
|
|
|
Lysosomal Acid Lipase Deficiency (LAL-D) Market Size
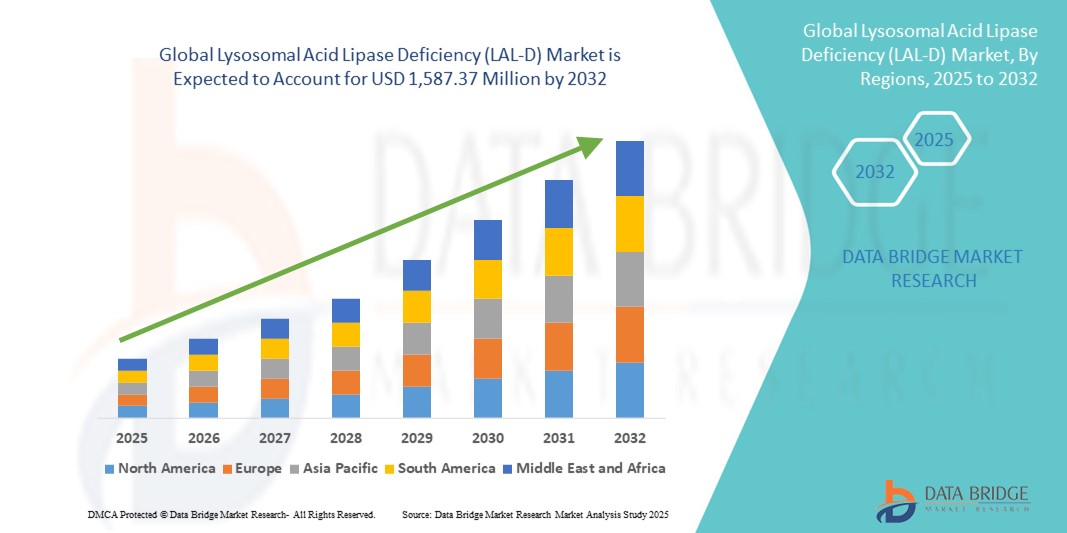
- The global lysosomal acid lipase deficiency (LAL-D) market size was valued at USD 740.52 million in 2024 and is expected to reach USD 1,587.37 million by 2032, at a CAGR of 10.00% during the forecast period
- The market growth is primarily driven by increasing awareness, early diagnosis initiatives, and advancements in enzyme replacement therapies for managing this rare genetic disorder
- Moreover, growing investment in rare disease research, regulatory incentives for orphan drugs, and a rising number of clinical trials are accelerating innovation in LAL-D treatment, collectively propelling market expansion and solidifying its growth trajectory
Lysosomal Acid Lipase Deficiency (LAL-D) Market Analysis
- Lysosomal acid lipase deficiency (LAL-D), a rare genetic disorder affecting lipid metabolism, is gaining clinical attention due to its severe health implications including progressive liver disease and premature atherosclerosis, driving demand for early diagnosis and targeted therapeutic interventions in both pediatric and adult populations
- The increasing demand for effective LAL-D treatments is primarily fueled by the availability of enzyme replacement therapies, rising awareness among healthcare professionals, and enhanced diagnostic capabilities through genetic testing and newborn screening programs
- North America dominated the LAL-D market with the largest revenue share of 47.2% in 2024, supported by strong healthcare infrastructure, established reimbursement frameworks, and the presence of key biopharmaceutical players investing in rare disease portfolios, with the U.S. leading in LAL-D clinical trial activity and patient support initiatives
- Asia-Pacific is expected to be the fastest growing region in the LAL-D market during the forecast period due to increasing awareness of rare diseases, improving healthcare access, and rising government and private sector investments in genetic and metabolic disorder diagnostics and treatment
- Enzyme replacement therapy segment dominated the LAL-D market with a market share of 61.8% in 2024, driven by its effectiveness in addressing the root enzyme deficiency, improving patient outcomes, and being the only approved therapeutic option currently available
Report Scope and Lysosomal Acid Lipase Deficiency (LAL-D) Market Segmentation
|
Attributes |
Lysosomal Acid Lipase Deficiency (LAL-D) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Lysosomal Acid Lipase Deficiency (LAL-D) Market Trends
Advancement in Enzyme Replacement Therapies and Genetic Screening
- A prominent and accelerating trend in the global LAL-D market is the advancement and refinement of enzyme replacement therapies (ERTs) coupled with the expansion of genetic screening programs. These innovations are significantly improving disease detection, timely intervention, and long-term disease management
- For instance, Kanuma (sebelipase alfa), the only FDA-approved ERT for LAL-D, has become the cornerstone of treatment, with ongoing efforts focused on enhancing its accessibility, dosing regimens, and outcomes through real-world studies and registry data
- The increasing adoption of next-generation sequencing (NGS) technologies has improved early diagnosis of LAL-D, particularly through newborn screening initiatives in select regions. These tools allow for rapid identification of LIPA gene mutations, enabling prompt treatment and improved prognosis
- Technological progress in diagnostic testing, such as dried blood spot (DBS) assays and multiplex screening platforms, is also driving earlier detection. This is particularly critical as delayed diagnosis often leads to irreversible organ damage in affected individuals
- Moreover, research is increasingly exploring combination therapies and novel delivery systems to improve the efficacy and bioavailability of enzyme treatments. Collaborative initiatives among academic institutions, biopharmaceutical companies, and patient advocacy groups are propelling innovation in LAL-D research
- This trend toward precision diagnostics and improved therapeutic options is reshaping expectations for rare metabolic disease management. Consequently, companies such as Alexion Pharmaceuticals are investing in awareness programs and global access strategies to expand the reach of LAL-D treatment, especially in underserved regions
- The growing demand for early, effective, and lifelong therapies for LAL-D is rapidly advancing the market, with increasing focus on newborn screening, personalized therapy, and comprehensive care pathways across developed and emerging economies
Lysosomal Acid Lipase Deficiency (LAL-D) Market Dynamics
Driver
Increased Awareness, Early Diagnosis, and Orphan Drug Incentives
- The rising awareness about lysosomal storage disorders and increasing efforts to educate healthcare professionals and patients have significantly contributed to earlier diagnosis and growing demand for specialized treatments such as enzyme replacement therapies
- For instance, global awareness campaigns and rare disease registries are helping identify undiagnosed LAL-D patients, particularly in pediatric populations, leading to earlier treatment initiation and better long-term outcomes
- Regulatory support in the form of orphan drug designations, fast-track approvals, and financial incentives is encouraging pharmaceutical companies to invest in rare disease research, particularly for conditions such as LAL-D with limited treatment options
- Enzyme replacement therapy (ERT), specifically sebelipase alfa, has demonstrated significant improvements in lipid profiles and liver function, driving its adoption among clinicians treating both children and adults with LAL-D
- In addition, advancements in genomics and growing availability of diagnostic technologies are facilitating early screening, while patient support programs and collaborations between health authorities and manufacturers are ensuring better access to therapy in key markets
Restraint/Challenge
Delayed Diagnosis, Limited Awareness in Emerging Regions, and High Cost of Therapy
- Despite advancements, delayed or missed diagnosis remains a critical challenge due to the non-specific symptoms of LAL-D and lack of awareness among general practitioners, especially in low- and middle-income countries
- For instance, despite regulatory approvals, access to ERT may be limited in regions without strong reimbursement systems or national rare disease frameworks, hindering market penetration and patient outcomes
- The rarity of the disease, limited inclusion in routine newborn screening panels, and overlapping clinical presentation with more common disorders often result in underdiagnosis or misdiagnosis, delaying effective intervention
- Moreover, the high cost of enzyme replacement therapy such as Kanuma, which can exceed hundreds of thousands of dollars per patient annually, presents a significant economic burden, particularly in countries with limited insurance coverage or public funding for rare disease treatments
- Overcoming these challenges requires a multi-faceted approach involving government support, expansion of screening programs, cost-reduction strategies by manufacturers, and increased investment in education and infrastructure to ensure equitable access to LAL-D diagnosis and treatment worldwide
Lysosomal Acid Lipase Deficiency (LAL-D) Market Scope
The market is segmented on the basis of type, treatment, route of administration, end-users, and distribution channel.
- By Type
On the basis of type, the lysosomal acid lipase deficiency (LAL-D) market is segmented into early onset Wolman disease and late onset cholesterol ester storage disease (CESD). The late onset CESD segment dominated the market with the largest market revenue share in 2024, driven by its higher prevalence and the relatively milder, chronic nature of the disease that allows for longer treatment duration and clinical management. Patients with CESD often experience progressive liver and cardiovascular complications, which are increasingly being addressed through enzyme replacement therapy and supportive lipid-lowering interventions.
The early onset Wolman disease segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by rising awareness, expanded newborn screening programs, and increasing use of early enzyme replacement therapy. Advancements in diagnostic genetics and improved survival outcomes in infants are also contributing to the segment's accelerating growth trajectory.
- By Treatment
On the basis of treatment, the lysosomal acid lipase deficiency (LAL-D) market is segmented into enzyme replacement therapy, lipid-modifying agents (statins), surgery, stem cell transplantation, and supportive care. The enzyme replacement therapy segment dominated the market with the largest market revenue share of 61.8% in 2024, primarily due to the widespread clinical adoption of sebelipase alfa (Kanuma), the only approved therapy targeting the underlying enzyme deficiency in LAL-D. Its demonstrated effectiveness in reducing lipid buildup and improving liver function across age groups reinforces its market leadership.
The lipid-modifying agents (statins) segment is expected to witness steady growth from 2025 to 2032, driven by their use in managing hyperlipidemia in late-onset CESD cases, particularly where enzyme therapy is delayed, unavailable, or used in combination. The accessibility and affordability of statins further support their ongoing utilization in clinical practice.
- By Route of Administration
On the basis of route of administration, the lysosomal acid lipase deficiency (LAL-D) market is segmented into oral, parenteral, and others. The parenteral segment dominated the market with the largest market revenue share in 2024, owing to the intravenous administration of sebelipase alfa, which is central to LAL-D treatment. Hospital-based infusions and physician-administered therapies ensure adherence and safety, reinforcing parenteral delivery as the dominant mode.
The oral segment is anticipated to grow steadily during the forecast period, supported by the use of statins and other oral lipid-lowering therapies, particularly in milder CESD cases. The convenience of oral administration and cost-effectiveness of these agents make them widely accessible in various healthcare settings.
- By End-Users
On the basis of end-users, the lysosomal acid lipase deficiency (LAL-D) market is segmented into clinics, hospitals, ambulatory surgical centres, and others. The hospital segment dominated the market with the largest market revenue share in 2024, due to the concentration of rare disease treatment infrastructure, infusion services, and multidisciplinary care teams required for LAL-D management. Hospitals also facilitate genetic diagnosis and specialist consultations, supporting their leading role.
The clinics segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by the decentralization of rare disease management, increased availability of outpatient genetic testing, and expanding role of metabolic specialists providing follow-up and supportive care outside hospital settings.
- By Distribution Channel
On the basis of distribution channel, the lysosomal acid lipase deficiency (LAL-D) market is segmented into direct tender, retail pharmacy, hospital pharmacy, and others. The hospital pharmacy segment dominated the market with the largest market revenue share in 2024, driven by institutional procurement and controlled dispensing of enzyme replacement therapy, which requires physician supervision and hospital infrastructure for administration.
The direct tender segment is expected to witness the fastest growth rate from 2025 to 2032, driven by public health initiatives and government contracts to procure orphan drugs for rare diseases. Direct tenders are particularly common in countries implementing national rare disease plans or reimbursement frameworks aimed at improving access to high-cost therapies such as sebelipase alfa.
Lysosomal Acid Lipase Deficiency (LAL-D) Market Regional Analysis
- North America dominated the lysosomal acid lipase deficiency (LAL-D) market with the largest revenue share of 47.2% in 2024, supported by strong healthcare infrastructure, established reimbursement frameworks, and the presence of key biopharmaceutical players investing in rare disease portfolios, with the U.S. leading in LAL-D clinical trial activity and patient support initiatives
- Patients and healthcare providers in the region benefit from greater awareness of lysosomal storage disorders, strong reimbursement systems, and access to FDA-approved therapies such as sebelipase alfa, promoting timely diagnosis and treatment
- This leadership position is further reinforced by the presence of major biopharmaceutical companies, ongoing clinical research, and coordinated efforts between healthcare authorities and advocacy organizations to enhance disease awareness and improve treatment accessibility across both pediatric and adult populations
U.S. Lysosomal Acid Lipase Deficiency (LAL-D) Market Insight
The U.S. lysosomal acid lipase deficiency (LAL-D) market captured the largest revenue share of 83% in 2024 within North America, fueled by high disease awareness, advanced diagnostic capabilities, and the widespread availability of sebelipase alfa under FDA approval. The presence of comprehensive newborn screening programs and rare disease registries supports early identification and timely treatment. In addition, strong payer coverage, active clinical research networks, and patient support programs from biopharmaceutical firms further reinforce the market’s strength in the U.S., making it the global leader in LAL-D treatment access and innovation.
Europe Lysosomal Acid Lipase Deficiency (LAL-D) Market Insight
The Europe lysosomal acid lipase deficiency (LAL-D) market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by growing public awareness of lysosomal storage disorders and favorable regulatory frameworks supporting orphan drugs. The European Medicines Agency’s continued incentives for rare disease treatments and the expansion of national rare disease plans are encouraging market growth. Moreover, collaborations between healthcare systems, advocacy groups, and pharmaceutical companies are facilitating better access to diagnostics and therapies across both Western and Eastern Europe.
U.K. Lysosomal Acid Lipase Deficiency (LAL-D) Market Insight
The U.K. lysosomal acid lipase deficiency (LAL-D) market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing investment in genetic screening programs and rare disease awareness initiatives. The National Health Service (NHS) continues to prioritize early detection and treatment of metabolic disorders, including LAL-D. In addition, improved access to enzyme replacement therapy through public healthcare and inclusion in rare disease strategies is driving adoption, especially among pediatric populations diagnosed via newborn screening.
Germany Lysosomal Acid Lipase Deficiency (LAL-D) Market Insight
The Germany lysosomal acid lipase deficiency (LAL-D) market is expected to expand at a considerable CAGR during the forecast period, supported by the country’s strong emphasis on innovation and advanced healthcare infrastructure. Germany is a hub for clinical research on rare metabolic diseases and has a well-established reimbursement system that supports access to high-cost therapies such as sebelipase alfa. The growing integration of genomics into clinical practice and early screening practices is enhancing diagnosis rates and enabling earlier treatment initiation.
Asia-Pacific Lysosomal Acid Lipase Deficiency (LAL-D)Market Insight
The Asia-Pacific lysosomal acid lipase deficiency (LAL-D) market is poised to grow at the fastest CAGR of 23.5% during the forecast period of 2025 to 2032, driven by improving healthcare infrastructure, growing awareness of rare diseases, and rising government investments in genetic diagnostics. Countries such as China, India, and Japan are increasing efforts in rare disease recognition, while the growing availability of international orphan drugs is expanding treatment access. Partnerships between global pharmaceutical firms and regional healthcare systems are also helping to increase diagnosis and therapy coverage in the region.
Japan Lysosomal Acid Lipase Deficiency (LAL-D) Market Insight
The Japan lysosomal acid lipase deficiency (LAL-D) market is gaining momentum due to the country’s advanced healthcare system, strong emphasis on early disease detection, and growing use of next-generation sequencing in pediatrics. Government-driven rare disease strategies and academic-clinical collaborations are accelerating early diagnosis. In addition, increasing adoption of enzyme replacement therapy in pediatric hospitals and specialist metabolic centers is contributing to the market's growth, with a notable focus on improving quality of life and long-term outcomes for patients.
India Lysosomal Acid Lipase Deficiency (LAL-D) Market Insight
The India lysosomal acid lipase deficiency (LAL-D) market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to growing genetic testing availability, improved access to healthcare in urban regions, and an expanding middle class. India's rare disease policy and inclusion of select metabolic disorders in public health programs are supporting early diagnosis and treatment access. With increasing collaborations between domestic healthcare providers and global pharmaceutical companies, awareness and access to LAL-D therapies are gradually expanding across major metropolitan centers.
Lysosomal Acid Lipase Deficiency (LAL-D) Market Share
The lysosomal acid lipase deficiency (LAL-D) industry is primarily led by well-established companies, including:
- Alexion Pharmaceuticals, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Morepen (India)
- LUPIN (India)
- Triveni Interchem Private Limited (India)
- Lannett (U.S.)
- Hetero Healthcare Limited (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Zydus Cadila (India)
- Sandoz International GmbH (Germany)
- BioCrick BioTech (China)
- Actiza Pharmaceutical Private Limited (India)
- Teva Pharmaceuticals USA, Inc. (U.S.)
- Glenmark Pharmaceutical Inc. (India)
- ANGLE BIO PHARMA (India)
- Accord Healthcare Limited (India)
- AstraZeneca (U.K.)
- Prudence Pharma Chem (India)
- Novadoz Pharmaceuticals (U.S.)
- Perrigo Company plc (Ireland)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.