Global Medical Device Adhesive Market
Market Size in USD Billion
CAGR :
% 
 USD
8.53 Billion
USD
16.91 Billion
2024
2032
USD
8.53 Billion
USD
16.91 Billion
2024
2032
| 2025 –2032 | |
| USD 8.53 Billion | |
| USD 16.91 Billion | |
|
|
|
|
Medical Device Adhesive Market Size
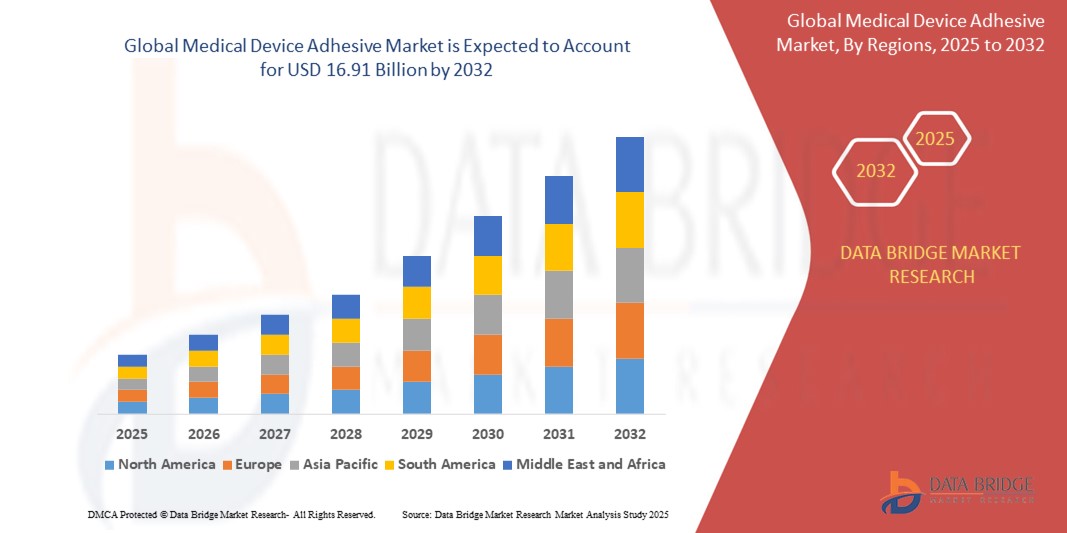
- The global medical device adhesive market was valued at USD 8.53 billion in 2024 and is expected to reach USD 16.91 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 7.9%, primarily driven by growing demand for minimally invasive procedures
- This growth is driven by factors such as the unique requirements of MIS necessitate adhesives that offer strong bonding, biocompatibility, and ease of removal. This demand drives innovation in adhesive formulations tailored for such applications
Medical device adhesive market Analysis
- Rising use of disposable medical devices, drives adhesive demand, particularly for syringes, diagnostic tools, and IV systems requiring safe, sterile bonding.
- Increased preference for minimally invasive surgeries fuels demand for high-performance, skin-friendly adhesives in wearable sensors, patches, and wound care devices.
- Manufacturers focus on developing biocompatible and eco-friendly adhesives to meet regulatory demands and patient safety, spurring innovation in water-based and solvent-free formulations.
Report Scope and Medical device adhesive market Segmentation
|
Attributes |
Medical Device Adhesive Polymers Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Medical device adhesive market Trends
“Shift Toward Biocompatible and Skin-Friendly Adhesive Formulations”
- There’s growing adoption of wearable healthcare devices such as glucose monitors, ECG patches, and insulin pumps. These require adhesives that stay on the skin for long durations without causing irritation. This has driven innovation in gentler, more breathable adhesive technologies.
- Companies like 3M and Henkel have developed low-trauma adhesives tailored for sensitive skin, particularly for pediatric, elderly, or long-term care patients.
- For instance, 3M's Extended Wear Medical Tapes, such as the 4576 and 4578, are designed for extended wear applications, offering secure adhesion to the skin for up to 21 days. These tapes are flexible, conformable, and minimize skin irritation, making them suitable for various medical and retail devices.
https://www.3mindia.in/3M/en_IN/p/d/v101544000/
- There is growing interest in adhesives derived from bio-based raw materials and those free from irritants like latex or acrylates. This not only supports sustainability goals but also reduces the risk of skin sensitization.
- As patient comfort and skin integrity become central to device performance and patient compliance, the demand for biocompatible adhesives is reshaping R&D and product development in the medical adhesive sector, positioning skin-friendly solutions as a competitive advantage.
Medical device adhesive market Dynamics
Driver
“Growing Demand for Minimally Invasive Procedures”
- Patients and healthcare providers increasingly favor MIS due to benefits like reduced recovery times, minimal scarring, and lower infection risks. These procedures often require specialized adhesives to secure devices such as catheters and endoscopes effectively.
- The unique requirements of MIS necessitate adhesives that offer strong bonding, biocompatibility, and ease of removal. This demand drives innovation in adhesive formulations tailored for such applications.
- The growth of wearable health monitors and implantable devices, which often involve minimally invasive placement, further amplifies the need for advanced adhesive solutions that can maintain performance over extended periods.
For instance,
- 3M's Tegaderm dressings are indeed widely used to cover insertion sites for IV catheters and central lines. These dressings are specifically designed for this purpose, offering features like securement, moisture management, and infection prevention, which are crucial for maintaining a clean and secure insertion site.
https://www.3m.com/3M/en_LB/p/d/b00035626/
- In conclusion, as the healthcare industry continues to prioritize patient comfort and procedural efficiency, the demand for specialized adhesives designed for MIS applications is expected to rise, fostering market growth.
Opportunity
“Expansion of Home Healthcare and Point-of-Care (PoC) Diagnostics”
- An aging global population and increasing prevalence of chronic conditions such as diabetes, cardiovascular disease, and respiratory disorders are boosting demand for home-based care. Medical adhesives are critical for securing sensors, patches, and other diagnostic components used outside clinical settings.
- Devices like lateral flow assays (e.g., COVID-19 self-tests), glucose meters, and rapid antigen tests require precise and reliable adhesive applications to bond layers of films, membranes, and housings. For instance, Scapa Healthcare and Avery Dennison supply adhesives specifically designed for high-volume diagnostic consumables.
- Home-use diagnostics must be simple to operate and affordable. Adhesives play a key role in device design by enabling streamlined assembly and durable performance without costly mechanical fasteners—particularly in single-use or disposable formats.
- The growing global emphasis on decentralized healthcare and PoC diagnostics creates a sustained and scalable opportunity for medical device adhesive manufacturers. Tailoring solutions for ease of use, sterility, and cost-efficiency will be critical for long-term growth in this segment.
Restraint/Challenge
“Regulatory Compliance and Approval Complexity”
- Medical device adhesives must meet stringent and often differing regulatory standards in the U.S. (FDA), Europe (MDR), and Asia (e.g., PMDA in Japan). This creates complexity for manufacturers aiming for global distribution.
- Adhesives used on or in the human body must undergo ISO 10993 testing to ensure they do not cause cytotoxicity, irritation, or sensitization. This testing is time-consuming and expensive, increasing development costs and time to market.
- Regulations evolve, such as the shift from MDD to MDR in the EU. Companies must constantly adapt formulations and documentation, even for long-established products. This strains resources and delays product launches.
- Navigating regulatory compliance remains a significant hurdle for the global medical device adhesive industry, affecting cost, innovation speed, and global market access.
Medical device adhesive market Scope
The market is segmented on the basis of resin type and application.
|
Segmentation |
Sub-Segmentation |
|
By Resin Type |
|
|
By Application |
|
Medical device adhesive market Regional Analysis
“North America is the Dominant Region in the Medical device adhesive market”
-
North America dominates the medical device adhesive market, due to presence of major key players is a major factor responsible for the growth of medical device adhesive market.
- The U.S. holds a significant share due to rising research and development activities to innovate with pharmaceutical drugs and equipment will further create lucrative growth opportunities for the medical device adhesive market.
“Asia-Pacific is Projected to Register the Highest Growth Rate”
-
Asia-Pacific region is expected to witness the highest growth rate in the medical device adhesive market, driven by rising medical tourism is indirectly inducing growth of medical device adhesive market in this region
- China is expected to lead the growth due to easy availability of low-cost labour and abundant raw materials will further grab many eyeballs of the manufacturers.
Medical device adhesive market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Medtronic (Ireland)
- 3M (U.S.)
- Baxter (U.S.)
- Carestream Health (U.S.)
- Henkel Adhesive Technologies India Private Limited (India)
- Dymax (U.S.)
- Dr. Hönle AG (Germany)
- H.B. Fuller Company (U.S.)
- Master Bond Inc. (U.S.)
- Permabond LLC (U.K.)
- EPOXY TECHNOLOGY, INC. (U.S.)
- Novachem Corporation Ltd (Canada)
- Incure Inc. (U.S.)
- Panacol-Elosol GmbH (Germany)
- Stryker (U.S.)
- Dentsply Sirona (U.S.)
- Bostik (France)
- Avery Dennison Corporation (U.S.)
- Dow (U.S.)
- Ashland (U.S.)
Latest Developments in Global Medical device adhesive market
-
In November 2022, Flexcon unveiled four new products aimed at the healthcare and pharmaceutical sectors.
- In October 2022, DuPont launched a Chinese-language regional website (liveo.dupont.cn) dedicated to its DuPont Liveo Healthcare Solutions brand. This initiative is designed to support the growing demand in China for premium, high-performance healthcare solutions.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Global Medical Device Adhesive Market, Supply Chain Analysis and Ecosystem Framework
To support market growth and help clients navigate the impact of geopolitical shifts, DBMR has integrated in-depth supply chain analysis into its Global Medical Device Adhesive Market research reports. This addition empowers clients to respond effectively to global changes affecting their industries. The supply chain analysis section includes detailed insights such as Global Medical Device Adhesive Market consumption and production by country, price trend analysis, the impact of tariffs and geopolitical developments, and import and export trends by country and HSN code. It also highlights major suppliers with data on production capacity and company profiles, as well as key importers and exporters. In addition to research, DBMR offers specialized supply chain consulting services backed by over a decade of experience, providing solutions like supplier discovery, supplier risk assessment, price trend analysis, impact evaluation of inflation and trade route changes, and comprehensive market trend analysis.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.