Global Medical Device Complaint Management Market
Market Size in USD Billion
CAGR :
% 
 USD
7.41 Billion
USD
12.59 Billion
2025
2033
USD
7.41 Billion
USD
12.59 Billion
2025
2033
| 2026 –2033 | |
| USD 7.41 Billion | |
| USD 12.59 Billion | |
|
|
|
|
Medical Device Complaint Management Market Size
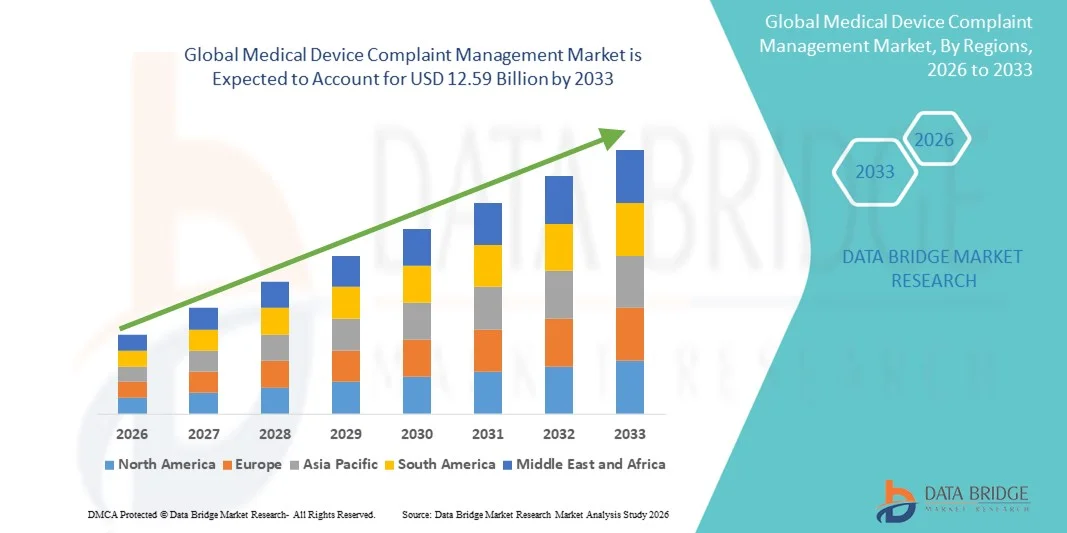
- The global medical device complaint management market size was valued at USD 7.41 billion in 2025 and is expected to reach USD 12.59 billion by 2033, at a CAGR of 6.86% during the forecast period
- The market growth is largely fueled by the increasing adoption of digital complaint management solutions and software that streamline the recording, tracking, investigation, and closure of complaints, replacing traditional paper‑based systems across medical device manufacturers
- Furthermore, stringent regulatory requirements, heightened focus on patient safety, and the need for efficient, compliant processes for handling device complaints are driving demand for integrated complaint management systems in both large enterprises and SMEs, establishing these solutions as critical components of medical device quality management systems
Medical Device Complaint Management Market Analysis
- Medical device complaint management solutions, which streamline the recording, tracking, investigation, and closure of device-related complaints, are increasingly vital components of quality management systems in both large enterprises and SMEs due to their ability to ensure regulatory compliance, enhance patient safety, and improve operational efficiency
- The escalating demand for medical device complaint management systems is primarily fueled by stringent regulatory requirements, growing emphasis on patient safety, and the need for digital, efficient, and compliant processes for handling complaints across the device lifecycle
- North America dominated the medical device complaint management market with the largest revenue share of 42.5% in 2025, driven by a well-established healthcare infrastructure, high adoption of digital solutions, and the presence of major medical device manufacturers, with the U.S. witnessing significant uptake of complaint management software as companies invest in automated and integrated quality management systems
- Asia-Pacific is expected to be the fastest growing region in the medical device complaint management market during the forecast period due to increasing medical device production, rising regulatory focus, and growing adoption of digital quality management solutions
- Complaints Log/Intake dominated the market with a share of 38.7% in 2025, driven by the critical need for accurate recording and tracking of device complaints for regulatory compliance and timely resolution
Report Scope and Medical Device Complaint Management Market Segmentation
|
Attributes |
Medical Device Complaint Management Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Medical Device Complaint Management Market Trends
Digital Transformation and AI-Enabled Complaint Analytics
- A significant and accelerating trend in the global medical device complaint management market is the adoption of digital platforms and AI-enabled analytics to streamline complaint logging, investigation, and resolution processes. This trend enhances operational efficiency and regulatory compliance across healthcare organizations
- For instance, companies such as Greenlight Guru provide cloud-based complaint management solutions integrated with AI analytics to detect recurring issues, track complaint trends, and predict potential product failures
- AI integration in complaint management enables features such as automated classification of complaints, intelligent prioritization for investigation, and predictive alerts for non-compliance. For instance, some Veeva systems leverage AI to flag anomalies in complaint patterns, helping regulatory teams respond proactively
- The integration of complaint management systems with enterprise quality management and ERP platforms facilitates centralized monitoring of product quality, risk mitigation, and regulatory reporting, improving overall organizational decision-making
- This trend towards automated, AI-driven, and interconnected complaint management solutions is reshaping expectations for medical device quality oversight. Consequently, companies such as MasterControl are enhancing their systems with AI and real-time reporting dashboards
- The demand for intelligent, fully digital complaint management systems is growing rapidly across both SMEs and large enterprises, as organizations prioritize compliance, efficiency, and proactive product safety monitoring
- Cloud-based deployment models are increasingly preferred, allowing manufacturers to access real-time complaint data across multiple sites, enabling faster decision-making and issue resolution
Medical Device Complaint Management Market Dynamics
Driver
Rising Regulatory Pressure and Focus on Patient Safety
- The increasing stringency of global medical device regulations and the growing emphasis on patient safety are significant drivers for the adoption of complaint management systems
- For instance, in March 2025, Medidata Solutions announced enhancements to its complaint management platform to align with FDA and EU MDR regulations, improving tracking and reporting of adverse events
- As manufacturers aim to comply with regulations such as FDA 21 CFR Part 11 and ISO 13485, automated complaint management solutions provide accurate, auditable, and timely reporting, reducing the risk of non-compliance
- Furthermore, the rising complexity of medical devices and product portfolios increases the need for structured complaint handling processes to maintain quality standards and protect patients
- Efficient complaint management software offers centralized data management, trend analysis, and faster resolution, enabling organizations to proactively address device issues while supporting regulatory compliance
- Increasing adoption of IoT-enabled medical devices creates more complex data streams, which drive the need for automated complaint tracking and analysis
- Growing focus on patient-centric care and proactive risk management encourages healthcare organizations to adopt advanced complaint management platforms to improve device reliability and trust
Restraint/Challenge
High Implementation Costs and Data Security Concerns
- The relatively high cost of deploying advanced digital complaint management systems, particularly for SMEs, can limit adoption in cost-sensitive regions
- For instance, smaller medical device manufacturers may find the licensing and integration costs of platforms such as Veeva or MasterControl challenging without immediate ROI
- Concerns around cybersecurity and protecting sensitive patient and complaint data pose another significant challenge, as these platforms store large volumes of confidential information
- For instance, reports of breaches in healthcare data systems have made some organizations cautious about transitioning to fully cloud-based complaint management solutions
- Overcoming these challenges through scalable pricing models, robust encryption, secure authentication, and staff training is critical to enable broader adoption and sustained growth in the market
- Lack of standardization in complaint reporting across different regions or regulatory bodies can create integration and compliance difficulties for global manufacturers
- Resistance to change from manual or legacy systems to automated digital platforms may slow adoption, especially among smaller enterprises with limited IT capabilities
Medical Device Complaint Management Market Scope
The market is segmented on the basis of service and application.
- By Service
On the basis of service, the medical device complaint management market is segmented into complaints log/intake, product surveillance & regulatory compliance, returned/non-returned product analysis, and resolve & closure. The Complaints Log/Intake segment dominated the market with the largest market revenue share of 38.7% in 2025, driven by the critical need for accurate recording and tracking of device complaints for regulatory compliance and timely resolution. Manufacturers rely on structured intake systems to ensure every complaint is logged correctly, minimizing the risk of missing adverse events. Centralized logging also supports trend analysis, helping organizations detect recurring issues early. The adoption of cloud-based intake systems allows real-time access across multiple sites, improving response time. Healthcare organizations also value this segment for its role in supporting audits and inspections. The integration with enterprise quality management systems further enhances operational efficiency and compliance reporting.
The Product Surveillance & Regulatory Compliance segment is expected to witness the fastest growth rate of 11.2% CAGR from 2026 to 2033, fueled by increasing regulatory scrutiny and global harmonization of medical device reporting standards. This segment involves continuous monitoring of product performance in the market and ensuring all complaint data meets compliance requirements. Advanced analytics and AI integration help manufacturers proactively detect risks and implement corrective actions. Regulatory authorities such as the FDA and EU MDR increasingly mandate robust post-market surveillance, driving adoption. Companies are investing in digital solutions to simplify compliance reporting and reduce the risk of penalties. This growth is also supported by expanding medical device production in emerging markets, where regulatory enforcement is strengthening.
- By Application
On the basis of application, the medical device complaint management market is segmented into small & medium enterprises (SMEs) and large enterprises. The Large Enterprise segment dominated the market with the largest market share of 59.3% in 2025, owing to the high volume of medical devices they manufacture and stringent regulatory requirements. Large organizations often operate across multiple geographies, requiring centralized complaint management systems to maintain consistency and compliance. They invest in integrated platforms with features such as AI analytics, automated workflows, and real-time dashboards to improve efficiency. Large enterprises also face higher scrutiny from regulators, making robust complaint management systems critical to avoid penalties. In addition, these systems help in strategic decision-making by analyzing complaint trends across products and regions. High adoption in this segment is also driven by enterprise-scale budgets and IT capabilities to implement sophisticated solutions.
The SME segment is expected to witness the fastest growth rate of 10.5% CAGR from 2026 to 2033, driven by increasing awareness of regulatory compliance and patient safety requirements. SMEs are increasingly adopting cloud-based and SaaS complaint management solutions to reduce upfront costs while ensuring compliance. These systems provide scalability, allowing smaller manufacturers to handle growing complaint volumes efficiently. SMEs are leveraging integrated analytics to identify product issues early and enhance customer trust. Adoption is further fueled by support from regional regulatory authorities and industry initiatives promoting best practices. The ease of deployment, lower maintenance costs, and compliance support make digital complaint management solutions highly attractive for SMEs.
Medical Device Complaint Management Market Regional Analysis
- North America dominated the medical device complaint management market with the largest revenue share of 42.5% in 2025, driven by a well-established healthcare infrastructure, high adoption of digital solutions, and the presence of major medical device manufacturers
- Organizations in the region highly value automated complaint management systems for their ability to ensure compliance with FDA regulations, ISO standards, and post-market surveillance obligations while enhancing patient safety and operational efficiency
- This widespread adoption is further supported by advanced IT infrastructure, high awareness of digital quality management solutions, and the growing focus on proactive risk management, establishing complaint management systems as a critical component of large-scale medical device operations
U.S. Medical Device Complaint Management Market Insight
The U.S. medical device complaint management market captured the largest revenue share of 83% in 2025 within North America, driven by the presence of leading medical device manufacturers and a stringent regulatory framework. Organizations are increasingly prioritizing digital complaint management systems to ensure compliance with FDA regulations and enhance patient safety. The growing adoption of cloud-based and AI-enabled platforms allows for real-time tracking, trend analysis, and proactive issue resolution. In addition, the integration of complaint management systems with enterprise quality management and post-market surveillance tools is significantly contributing to the market expansion. The robust healthcare infrastructure and high IT capabilities further support widespread adoption.
Europe Medical Device Complaint Management Market Insight
The Europe medical device complaint management market is projected to expand at a substantial CAGR throughout the forecast period, driven by stringent EU MDR regulations and the rising need for patient safety and product quality compliance. The growing number of medical device manufacturers and increasing adoption of digital quality management systems are fostering market growth. European organizations are leveraging complaint management platforms to ensure compliance, perform trend analysis, and manage post-market surveillance efficiently. Demand is increasing across large enterprises and SMEs, particularly in healthcare hubs such as Germany, France, and the Netherlands. Regulatory scrutiny and proactive risk management are key factors encouraging adoption.
U.K. Medical Device Complaint Management Market Insight
The U.K. medical device complaint management market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by the increasing adoption of digital quality management solutions and growing awareness of regulatory compliance requirements. Organizations are seeking efficient complaint tracking and resolution systems to mitigate risks and maintain product safety. Concerns regarding potential recalls and regulatory penalties are driving both SMEs and large manufacturers to implement automated complaint management platforms. In addition, the U.K.’s advanced healthcare infrastructure, robust IT ecosystem, and strong focus on patient safety are expected to continue stimulating market growth.
Germany Medical Device Complaint Management Market Insight
The Germany medical device complaint management market is expected to expand at a considerable CAGR during the forecast period, driven by a combination of stringent regulatory requirements, rising medical device production, and an emphasis on product quality. German manufacturers are increasingly adopting AI-enabled and cloud-based complaint management systems to streamline processes, enhance compliance, and improve operational efficiency. The integration of these systems with enterprise quality management platforms allows organizations to perform proactive risk analysis and regulatory reporting. Germany’s well-developed infrastructure and focus on innovation further support adoption in both SMEs and large enterprises.
Asia-Pacific Medical Device Complaint Management Market Insight
The Asia-Pacific medical device complaint management market is poised to grow at the fastest CAGR of 12.5% during 2026–2033, fueled by increasing medical device manufacturing, expanding regulatory focus, and rising adoption of digital quality management solutions in countries such as China, India, and Japan. Growing awareness of patient safety, regulatory compliance, and proactive risk management is driving demand for automated complaint management platforms. Cloud-based and SaaS solutions are facilitating adoption among SMEs and large enterprises alike. The expansion of healthcare infrastructure and rising investment in digital healthcare systems are also key growth drivers.
Japan Medical Device Complaint Management Market Insight
The Japan medical device complaint management market is gaining momentum due to the country’s advanced healthcare ecosystem, strict regulatory oversight, and the adoption of high-tech quality management solutions. Manufacturers are increasingly implementing digital platforms to monitor, analyze, and resolve complaints efficiently. Integration with post-market surveillance systems and AI-enabled analytics helps in proactive risk management and compliance reporting. The focus on patient safety and regulatory adherence drives adoption in both large enterprises and SMEs. In addition, Japan’s aging population and the expansion of connected medical devices are expected to further spur demand for complaint management solutions.
India Medical Device Complaint Management Market Insight
The India medical device complaint management market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to the country’s expanding medical device manufacturing sector, rapid regulatory modernization, and growing focus on patient safety. Digital complaint management solutions are increasingly adopted by SMEs and large enterprises to streamline compliance, improve product quality, and enhance operational efficiency. Government initiatives promoting healthcare digitalization and the rise of smart hospitals are further fueling market growth. The availability of affordable cloud-based platforms and increasing awareness of global regulatory standards are key factors propelling the market in India.
Medical Device Complaint Management Market Share
The Medical Device Complaint Management industry is primarily led by well-established companies, including:
- MasterControl Solutions, Inc. (U.S.)
- Greenlight Guru (U.S.)
- AssurX, Inc. (U.S.)
- IQVIA Inc. (U.S.)
- Veeva Systems Inc. (U.S.)
- Oracle (U.S.)
- SAP SE (Germany)
- ComplianceQuest Inc. (U.S.)
- Qualio (U.S.)
- Siemens Healthineers AG (Germany)
- Wipro Limited (India)
- SAS Institute Inc. (U.S.)
- Intellect Inc. (U.S.)
- MetricStream Inc. (U.S.)
- Arena Solutions, Inc. (U.S.)
- Ennov Group (France)
- Ideagen plc (U.K.)
- Xybion Corporation (U.S.)
- Parexel International Corporation (U.S.)
- BizzMine (Belgium)
What are the Recent Developments in Global Medical Device Complaint Management Market?
- In May 2025, Honeywell’s TrackWise Recall Management was launched, a cloud‑based, AI‑assisted recall software designed to help life sciences and medical device manufacturers proactively manage product recalls and enhance patient safety
- In May 2025, Smarteeva announced API integration support for the FDA’s Electronic Submissions Gateway Next Generation (ESG NextGen), enabling faster and automated regulatory data exchange for complaint and adverse event reporting
- In November 2024, Veeva Systems launched Veeva Vault Complaints, an integrated complaint management solution tailored for medical device manufacturers, offering automated workflows and seamless integration with quality systems to boost regulatory compliance and operational efficiency
- In November 2024, Becton, Dickinson and Company (BD) received a warning letter from the U.S. Food and Drug Administration (FDA) citing deficiencies in complaint handling and quality systems for its Pyxis MedStation ES medication dispensing system, including failure to report complaints in a timely manner and other complaint management breaches under regulatory requirements
- In April 2024, FTI Consulting published a major industry insight titled “Elevating Medical Device Complaint Management: Strategies for Enhanced Resolution and Mitigation of Complaints”, emphasizing the critical role of integrated complaint handling in global quality management systems and its impact on compliance and operational performance
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.