Global Medical Device Engineering Market
Market Size in USD Billion
CAGR :
% 
 USD
7.66 Billion
USD
11.83 Billion
2024
2032
USD
7.66 Billion
USD
11.83 Billion
2024
2032
| 2025 –2032 | |
| USD 7.66 Billion | |
| USD 11.83 Billion | |
|
|
|
|
Medical Device Engineering Market Analysis
The medical device engineering market is experiencing robust growth, driven by advancements in healthcare technology, increased investment in R&D, and rising demand for innovative medical solutions. With a market size projected to grow at a CAGR of over 5.8%, the industry benefits from the development of minimally invasive devices, wearable health technologies, and diagnostic tools powered by AI and IoT. Key factors influencing this growth include the aging global population and the rising prevalence of chronic diseases, which necessitate advanced medical devices for effective management. Regulatory changes, such as the EU's MDR (Medical Device Regulation) and stringent FDA requirements, are driving companies to invest in high-quality engineering solutions to ensure compliance and safety. Technological advancements, including 3D printing, robotics, and smart materials, are reshaping medical device engineering. These innovations enable faster prototyping, cost efficiency, and enhanced device functionality. The Asia-Pacific region is emerging as a significant contributor, driven by lower manufacturing costs and a growing healthcare infrastructure. Challenges like high R&D costs and regulatory hurdles remain, but the demand for cutting-edge medical devices in diagnostics, treatment, and monitoring systems ensures a positive trajectory for the industry. The integration of digital health solutions will further propel this market.
Medical Device Engineering Market Size
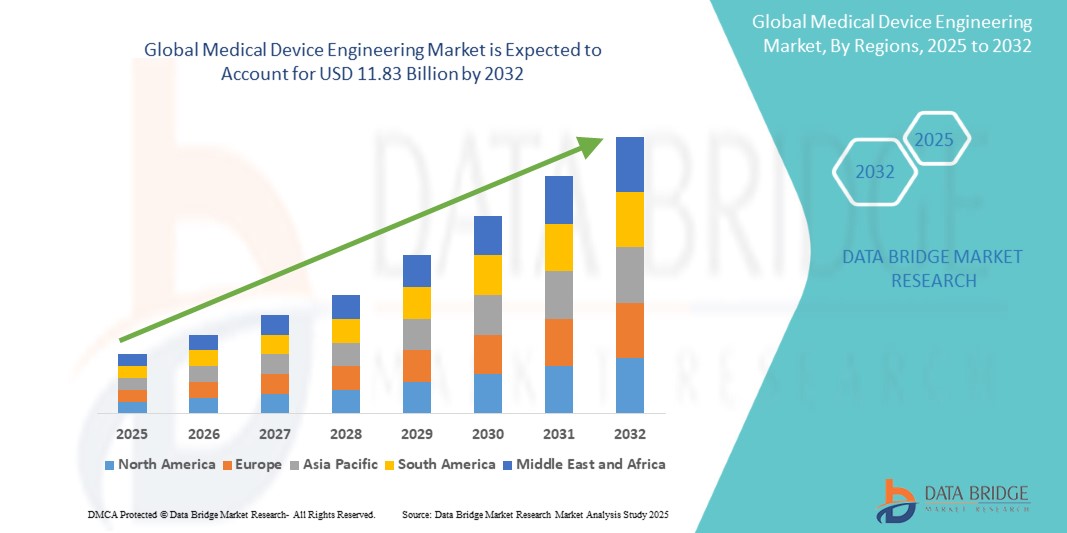
Global medical device engineering market size was valued at USD 7.66 billion in 2024 and is projected to reach USD 11.83 billion by 2032, with a CAGR of 5.8% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Medical Device Engineering Market Trends
“Artificial Intelligence (AI) and Machine Learning (ML) Integration in Medical Devices”
One of the most transformative trends in medical device engineering market is the integration of Artificial Intelligence (AI) and Machine Learning (ML) capabilities. AI and ML are enabling medical devices to perform more advanced functions such as diagnostics, data analysis, and personalized treatment recommendations, which are paving the way for a new generation of “smart” medical devices
AI-driven medical devices can analyze complex datasets and identify patterns that may not be apparent to human operators. For instance, AI algorithms embedded in imaging devices such as MRI and CT scanners help radiologists detect abnormalities such as tumors, fractures, or blood clots more accurately and at an earlier stage. This is helping healthcare providers make quicker and more accurate diagnoses, leading to better patient outcomes. AI-enabled devices can create individualized treatment plans by analyzing a patient’s unique physiological data, medical history, and genetic information. This is particularly valuable in fields like oncology, where treatments can be tailored to the specific genetic profile of a patient’s tumor, improving the effectiveness of therapies.
Report Scope and Medical Device Engineering Market Segmentation
|
Attributes |
Agriculture Rollers Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
L&T Technology Services Limited (India), Infosys Limited (India), HCL Technologies Limited (India), Cyient (India), Wipro (India), Tech Mahindra Limited (India), TATA Consultancy Services Limited (India), FLEX LTD (U.S.), Capgemini (France), Embien Technologies India Pvt Ltd. (India), Alten Group (France), Accenture (Ireland), Consonance (Poland), Althea Group (U.S.), MED INSTITUTE (U.S.), Saraca Solutions Private Limited (India), Nemedio Inc. (U.S.), Sternum (Israel), Medcrypt (U.S.), MCRA, LLC (U.S.), North American Science Associates, LLC (U.S.), MedQtech (Sweden), Veranex (U.S.), Ontogen Medtech LLC (U.S.), Seisa Media (U.S.), and Simplexity Product Development (U.S.). |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Medical Device Engineering Market Definition
Medical device engineering is a multidisciplinary field focused on the design, development, and manufacturing of devices used for medical purposes, such as diagnosis, treatment, monitoring, or prevention of diseases. It integrates principles of mechanical, electrical, software, and biomedical engineering to create innovative and reliable devices, ranging from wearable health monitors and imaging systems to minimally invasive surgical tools and prosthetics. This field emphasizes compliance with stringent regulatory standards, safety, and user-centered design to ensure efficacy and patient comfort. Advances in materials science, robotics, and digital health technologies, like AI and IoT, are reshaping medical device engineering for improved healthcare outcomes.
Medical Device Engineering Market Dynamics
Drivers
- Increasing Prevalence of Chronic Diseases
The rising prevalence of chronic diseases such as cardiovascular disease, diabetes, cancer, and respiratory disorders is a major driver for the medical device engineering market. Chronic diseases are long-term, often lifestyle-related conditions that require ongoing monitoring, management, and treatment. As these diseases become more common due to factors such as aging populations, urbanization, and sedentary lifestyles, there is a growing need for medical devices that can aid in diagnostics, monitoring, and treatment. Conditions such as diabetes and cardiovascular diseases necessitate constant monitoring. Devices such as glucose monitors, heart rate monitors, and even wearable ECGs have become essential for patients, allowing for real-time health tracking and quick response to adverse events.
For instance, according to 2021 International Diabetes Federation (IDF) report, 10.5% of the adult population (20-79 years) has diabetes, with almost half unaware that they are living with the condition. By 2045, IDF projections show that 1 in 8 adults, approximately 783 million, will be living with diabetes, an increase of 46%.
The demand for medical devices used in managing chronic diseases has led to innovations in portable, user-friendly, and cost-effective devices, making healthcare more accessible. For instance, the development of connected devices that transmit data to healthcare providers in real time is helping to address chronic disease management at both the patient and system levels. This trend is expected to increase, contributing significantly to the growth of the medical device engineering market.
- Technological Advancements in Medical Devices
Rapid technological innovation is a powerful driver in the medical device engineering market, reshaping devices and enhancing their capabilities. Advances in fields such as artificial intelligence, robotics, 3D printing, and biocompatible materials are enabling the development of sophisticated medical devices that offer greater precision, automation, and adaptability to individual patient needs. The adoption of these advanced technologies is enabling the medical device market to meet growing demands for more effective, efficient, and patient-centered solutions. Companies investing in these technologies are poised to capture market share by offering innovative devices that are easier for clinicians to use and provide enhanced clinical benefits. For instance, Philips leverages AI in its diagnostic imaging devices, such as the IntelliSpace AI Workflow Suite. This platform uses AI to enhance imaging quality, speed up diagnostic workflows, and assist in earlier, more accurate diagnosis. It's designed for radiologists to streamline imaging operations and provide improved patient outcomes. As healthcare systems worldwide increasingly adopt these advanced solutions, the medical device engineering market is expected to experience robust growth driven by technological progress.
Opportunities
- Expansion in Wearable and Remote Monitoring Devices
With the rise in telemedicine and demand for proactive health management, wearable and remote monitoring devices present a substantial opportunity in the medical device engineering market. These devices, including smartwatches, continuous glucose monitors, and cardiac monitors, allow for real-time health monitoring outside of traditional clinical settings. The ability to track vital signs such as heart rate, oxygen levels, and glucose provides continuous patient data and empowers individuals to manage their health more effectively. Wearable devices offer immense value for patients managing chronic conditions like diabetes, hypertension, and heart disease. With increasing awareness of preventive healthcare and growing investment in wearable technologies, this segment is anticipated to see significant growth. According to market research, the wearable medical device segment is expected to grow at a high CAGR over the next decade, contributing heavily to the overall medical device market’s expansion.
For instance, in August 2024, Dexcom announced its new over-the-counter continuous glucose monitor Stelo is officially available for purchase in the U.S. The Stelo Glucose Biosensor System is an over-the-counter (OTC) integrated Continuous Glucose Monitor (iCGM) intended to continuously measure, record, analyze, and display glucose values in people 18 years and older not on insulin. The Stelo Glucose Biosensor System helps to detect normal (euglycemic) and low or high (dysglycemic) glucose levels. The Stelo Glucose Biosensor System may also help the user better understand how lifestyle and behavior modification, including diet and exercise, impact glucose excursion. Thus, the increase in availability of wearable and remote monitoring devices is expected to drives the growth of the market during the forecast period.
- Growing Demand for Minimally Invasive Surgical Devices
The shift toward minimally invasive procedures presents another substantial opportunity for medical device engineering. Minimally invasive surgical (MIS) devices, including laparoscopic instruments, robotic-assisted surgery tools, and endoscopic devices, reduce recovery time, cause less trauma, and minimize postoperative complications. The market for MIS devices is expanding as patients and healthcare providers seek options that shorten hospital stays and improve patient outcomes. MIS techniques involve smaller incisions, which reduce the risk of infection, pain, and scarring. Robotic-assisted surgery, for instance, enables greater precision in complex procedures such as cardiac and orthopedic surgeries, improving outcomes and recovery times.
This trend is being bolstered by technological advancements in robotics, imaging, and surgical tools. The MIS devices market is expected to see robust growth, with projections indicating high demand, particularly in orthopedic, cardiovascular, and gastrointestinal applications. The opportunity is also enhanced by an aging population that often requires surgical interventions for chronic conditions, which MIS techniques can address with fewer complications and faster recoveries. For instance, Stryker’s Mako System is a robotic-assisted platform that enables orthopedic surgeons to perform precise, minimally invasive knee and hip replacement surgeries. The Mako System integrates 3D imaging and robotic arms, allowing for improved precision and patient-specific surgical planning. The introduction of this type of devices boost the growth of market during the forecast period.
Restraints/Challenges
- Regulatory Compliance and Approval Delays
One of the most significant challenges facing the medical device engineering industry is navigating complex and stringent regulatory requirements. To obtain regulatory approval, medical devices must undergo rigorous testing and clinical trials to demonstrate safety and efficacy. This process can be time-consuming and expensive, often leading to delays in product launches and market entry. Moreover, the regulatory landscape is constantly evolving, with new regulations and standards being introduced. This can create additional challenges for manufacturers, who must stay updated on the latest requirements and adapt their products accordingly.
Furthermore, the regulatory landscape can vary significantly across different countries. Obtaining approvals in multiple markets can be a complex and costly process. This can hinder the global distribution of medical devices and limit market access.
- High Initial Costs of Medical Device
The high cost of medical devices is a significant barrier to the growth of the medical device engineering market. Expensive materials, complex manufacturing processes, and stringent regulatory requirements contribute to the overall cost of these devices. As a result, healthcare providers, especially in developing countries, may struggle to afford advanced medical technologies, limiting market adoption. Additionally, high prices can deter innovation from smaller companies that lack the financial resources to invest in R&D and comply with regulatory standards. This restricts the accessibility and scalability of new technologies.
For instance, according to the American Council on Science and Health, the cost of medical devices are rarely mentioned. It represents about 5% of healthcare spending, or roughly USD120 billion in 2017. Therefore, the high cost of medical devices restraints to the growth of the medical device engineering market.
Medical Device Engineering Market Scope
The market is segmented on the basis of service type and device type. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Service Type
- Product Innovation & Design/Industrial Design Services
- Prototyping Services
- Electronics Engineering Services
- Software Development & Testing Services
- Connectivity And Mobility Services
- Cybersecurity Services
- Product Testing Services
- Regulatory Consulting Services
- Product Support & Maintenance Service
Device Type
- Diagnostic Imaging Equipment
- Surgical Equipment
- Patient Monitoring Devices & Life Support Devices
- Medical Lasers
- IVD Devices
- Other Medical Devices
Medical Device Engineering Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, device type, service type as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to advancements in medical device technology and high healthcare expenditure, presence of key players. Additionally, the high cost of medical device and high adoption rate of medical device are expected to further fuel the market's growth.
Asia-Pacific is expected to be the fastest growing due to the region's rapidly increasing population. Government initiatives and investments aimed at modernizing healthcare sector, along with increasing awareness among people about the benefits of advanced medical devices, surge in start-up activity in medical devices are contributing to the market's expansion.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Medical Device Engineering Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Medical Device Engineering Market Leaders Operating in the Market Are:
- L&T Technology Services Limited (India)
- Infosys Limited (India)
- HCL Technologies Limited (India)
- Cyient (India)
- Wipro (India)
- Tech Mahindra Limited (India)
- TATA Consultancy Services Limited (India)
- FLEX LTD (U.S.)
- Capgemini (France)
- Embien Technologies India Pvt Ltd. (India)
- Alten Group (France)
- Accenture (Ireland)
- Consonance (Poland)
- Althea Group (U.S.)
- MED INSTITUTE (U.S.)
- Saraca Solutions Private Limited (India)
- Nemedio Inc. (U.S.)
- Sternum (Israel)
- Medcrypt (U.S.)
- MCRA, LLC (U.S.)
- North American Science Associates, LLC (U.S.)
- MedQtech (Sweden)
- Veranex (U.S.)
- Ontogen Medtech LLC (U.S.)
- Seisa Media (U.S.)
- Simplexity Product Development (U.S.)
Latest Developments in Medical Device Engineering Market
- In November 2023, L&T Technology Services Limited (LTTS) (India) partnered with NVIDIA Corporation (U.S.) to develop software-defined architectures for medical devices, specifically for endoscopy, aimed at improving image quality and product scalability
- In October 2023, Alten Group (France) acquired East Japan Institute of Technology Co., Ltd. (Japan). This acquisition supports Alten’s strategy to strengthen its engineering capabilities in Japan and reach a significant market presence, establishing itself as a leading engineering services provider in the region
- In January 2022, HCL Technologies Limited (India) acquired Starschema (U.S.), a move that enhances HCL's digital engineering capabilities, especially in dassssta engineering, and broadens its footprint in Central and Eastern Europe
- In September 2024, Vision Engineering, a U.K. designer, manufacturer, and exporter of ergonomic microscopes and measuring systems, launched OPTA, a cost-effective entry-level product for its patented optical stereo microscope technology. Available for less than $1,200, with a choice of three stands and two lenses, OPTA redefines the entry point for users to benefit from Vision Engineering’s image quality, ergonomic design, and ease of use. This product aims to enhance image quality and ergonomics, making advanced microscopy more accessible. The launch of this type of devices boosts the growth of medical device engineering market
- In June 2022, Siemens Healthineers introduces Symbia Pro.specta, a single photon emission computed tomography/computed tomography (SPECT/CT) system with CE mark and Food and Drug Administration (FDA) clearance that has advanced SPECT and CT imaging technologies. Capabilities include a low-dose CT of up to 64 slices for impressive detail, automatic SPECT motion correction for additional image clarity, and an intuitive and automated workflow to guide the user through the entire decision-making process of the examination
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1. INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL MEDICAL DEVICE ENGINEERING MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2. MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL MEDICAL DEVICE ENGINEERING MARKETSIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL MEDICAL DEVICE ENGINEERING MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3. MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4. EXECUTIVE SUMMARY
5. PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6. INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7. INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8. COST ANALYSIS BREAKDOWN
9. TECHNONLOGY ROADMAP
10. INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11. REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12. REIMBURSEMENT FRAMEWORK
13. OPPUTUNITY MAP ANALYSIS
14. VALUE CHAIN ANALYSIS
15. HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.10 ECONOMIC DEVELOPMENT
16. GLOBAL MEDICAL DEVICE ENGINEERING MARKET, BY SERVICE TYPE
16.1 OVERVIEW
16.2 PRODUCT DESIGN & DEVELOPMENT
16.2.1 CONCEPTUALIZATION & IDEATION
16.2.2 INDUSTRIAL & MECHANICAL DESIGN
16.2.3 USER-CENTERED DESIGN (UCD) & ERGONOMICS
16.2.4 MATERIALS & COMPONENT SELECTION
16.2.5 FINITE ELEMENT ANALYSIS (FEA) & SIMULATION
16.2.6 DESIGN FOR MANUFACTURING (DFM)
16.3 PROTOTYPING & TESTING
16.3.1 RAPID PROTOTYPING (3D PRINTING, CNC, INJECTION MOLDING)
16.3.2 MECHANICAL, ELECTRICAL & THERMAL TESTING
16.3.3 PRECLINICAL TESTING & VALIDATION
16.3.4 BIOCOMPATIBILITY & TOXICITY TESTING
16.3.5 FAILURE MODE & EFFECTS ANALYSIS (FMEA)
16.4 MANUFACTURING & SUPPLY CHAIN MANAGEMENT
16.4.1 CONTRACT MANUFACTURING (OEM & ODM)
16.4.2 COMPONENT SOURCING & SUPPLY CHAIN OPTIMIZATION
16.4.3 CLEANROOM & STERILE MANUFACTURING
16.4.4 LEAN MANUFACTURING & SIX SIGMA PRACTICES
16.4.5 QUALITY CONTROL & ASSURANCE (ISO 13485, GMP)
16.5 REGULATORY COMPLIANCE & CONSULTING
16.5.1 RISK MANAGEMENT & SAFETY ASSESSMENTS
16.5.2 CLINICAL & REGULATORY STRATEGY CONSULTING
16.5.3 REGULATORY SUBMISSIONS (FDA, CE MARKING, ISO CERTIFICATION)
16.5.4 POST-MARKET SURVEILLANCE & COMPLIANCE AUDITS
16.5.5 LEGAL & ETHICAL CONSIDERATIONS
16.6 EMBEDDED SYSTEMS & SOFTWARE DEVELOPMENT
16.7 OTHER
17. GLOBAL MEDICAL DEVICE ENGINEERING MARKET, BY DEVICE TYPE
17.1 OVERVIEW
(NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS OF PRODUCT)
17.2 DIAGNOSTIC DEVICES
17.2.1 IMAGING SYSTEMS
17.2.1.1. MAGNETIC RESONANCE IMAGING (MRI)
17.2.1.1.1. OPEN MRI
17.2.1.1.2. CLOSED MRI
17.2.1.2. COMPUTED TOMOGRAPHY (CT) SCANNERS
17.2.1.2.1. LOW-SLICE CT (1-16 SLICES)
17.2.1.2.2. MID-SLICE CT (32-64 SLICES)
17.2.1.2.3. HIGH-SLICE CT (128+ SLICES)
17.2.1.2.4. CONE BEAM CT (CBCT)
17.2.1.2.5. SPECTRAL CT
17.2.1.3. ULTRASOUND DEVICES
17.2.1.3.1. 2D ULTRASOUND
17.2.1.3.2. 3D/4D ULTRASOUND
17.2.1.3.3. DOPPLER ULTRASOUND
17.2.1.3.4. PORTABLE & HANDHELD ULTRASOUND
17.2.1.3.5. HIGH-INTENSITY FOCUSED ULTRASOUND (HIFU)
17.2.1.4. X-RAY SYSTEMS
17.2.1.4.1. ANALOG X-RAY
17.2.1.4.2. DIGITAL RADIOGRAPHY (DR)
17.2.1.4.3. COMPUTED RADIOGRAPHY (CR)
17.2.1.4.4. FLUOROSCOPY SYSTEMS
17.2.1.5. POSITRON EMISSION TOMOGRAPHY (PET) SCANNERS
17.2.1.5.1. PET-CT SYSTEMS
17.2.1.5.2. PET-MRI SYSTEMS
17.2.1.5.3. STANDALONE PET SCANNERS
17.2.2 IN-VITRO DIAGNOSTIC (IVD) EQUIPMENT
17.2.2.1. MOLECULAR DIAGNOSTICS
17.2.2.1.1. POLYMERASE CHAIN REACTION (PCR)
17.2.2.1.2. NEXT-GENERATION SEQUENCING (NGS)
17.2.2.1.3. DNA MICROARRAYS
17.2.2.1.4. ISOTHERMAL NUCLEIC ACID AMPLIFICATION
17.2.2.2. IMMUNOASSAYS
17.2.2.2.1. ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA)
17.2.2.2.2. CHEMILUMINESCENCE IMMUNOASSAY (CLIA)
17.2.2.2.3. RADIOIMMUNOASSAY (RIA)
17.2.2.3. HEMATOLOGY ANALYZERS
17.2.2.3.1. 3-PART HEMATOLOGY ANALYZERS
17.2.2.3.2. 5-PART HEMATOLOGY ANALYZERS
17.2.2.3.3. POINT-OF-CARE HEMATOLOGY DEVICES
17.2.2.4. POINT-OF-CARE TESTING DEVICES
17.2.2.4.1. GLUCOSE MONITORING SYSTEMS
17.2.2.4.2. RAPID ANTIGEN/ANTIBODY TESTS
17.2.2.4.3. HOME PREGNANCY & FERTILITY KITS
17.2.2.4.4. INFECTIOUS DISEASE RAPID TESTS
17.3 THERAPEUTIC DEVICES
17.3.1 SURGICAL INSTRUMENTS & ROBOTICS
17.3.1.1. MINIMALLY INVASIVE SURGERY (MIS) DEVICES
17.3.1.1.1. LAPAROSCOPIC INSTRUMENTS
17.3.1.1.2. ENDOSCOPIC INSTRUMENTS
17.3.1.1.3. ARTHROSCOPIC DEVICES
17.3.1.1.4. ROBOTIC-ASSISTED MIS SYSTEMS
17.3.1.2. ROBOT-ASSISTED SURGERY (RAS) SYSTEMS
17.3.1.2.1. ORTHOPEDIC SURGERY ROBOTS
17.3.1.2.2. NEUROSURGERY ROBOTS
17.3.1.2.3. UROLOGY & GYNECOLOGY SURGERY ROBOTS
17.3.2 IMPLANTABLE MEDICAL DEVICES
17.3.2.1. ORTHOPEDIC IMPLANTS
17.3.2.1.1. HIP IMPLANTS
17.3.2.1.2. KNEE IMPLANTS
17.3.2.1.3. SPINAL IMPLANTS
17.3.2.1.4. TRAUMA FIXATION DEVICES
17.3.2.2. CARDIOVASCULAR IMPLANTS
17.3.2.2.1. PACEMAKERS
17.3.2.2.2. IMPLANTABLE CARDIOVERTER DEFIBRILLATORS (ICDS)
17.3.2.2.3. HEART VALVES
17.3.2.2.4. CORONARY & PERIPHERAL STENTS
17.3.2.3. NEUROSTIMULATION DEVICES
17.3.2.3.1. DEEP BRAIN STIMULATORS (DBS)
17.3.2.3.2. SPINAL CORD STIMULATORS (SCS)
17.3.2.3.3. VAGUS NERVE STIMULATORS (VNS)
17.3.2.3.4. SACRAL NERVE STIMULATORS
17.3.2.4. COCHLEAR & AUDITORY IMPLANTS
17.3.2.4.1. COCHLEAR IMPLANTS
17.3.2.4.2. BONE-ANCHORED HEARING AIDS (BAHA)
17.3.3 DRUG DELIVERY SYSTEMS
17.3.3.1. SMART INFUSION PUMPS
17.3.3.1.1. LARGE VOLUME PUMPS (LVP)
17.3.3.1.2. SYRINGE INFUSION PUMPS
17.3.3.1.3. PATIENT-CONTROLLED ANALGESIA (PCA) PUMPS
17.3.3.1.4. INSULIN PUMPS
17.3.3.2. SMART INHALERS
17.3.3.2.1. METERED-DOSE INHALERS (MDIS)
17.3.3.2.2. DRY POWDER INHALERS (DPIS)
17.3.3.2.3. NEBULIZERS
17.4 PATIENT MONITORING DEVICES
17.4.1 WEARABLE MEDICAL DEVICES
17.4.1.1. SMARTWATCHES & FITNESS TRACKERS
17.4.1.1.1. HEART RATE MONITORING
17.4.1.1.2. BLOOD OXYGEN (SPO2) MONITORING
17.4.1.1.3. ECG MONITORING
17.4.1.2. CONTINUOUS GLUCOSE MONITORS (CGM)
17.4.1.2.1. REAL-TIME CGM (RTCGM)
17.4.1.2.2. INTERMITTENTLY SCANNED CGM (ISCGM)
17.4.2 REMOTE PATIENT MONITORING (RPM) SYSTEMS
17.4.2.1. CARDIAC MONITORING DEVICES
17.4.2.1.1. HOLTER MONITORS
17.4.2.1.2. IMPLANTABLE LOOP RECORDERS
17.4.2.1.3. WIRELESS ECG
17.4.2.2. RESPIRATORY MONITORING DEVICES
17.4.2.2.1. PULSE OXIMETERS
17.4.2.2.2. CAPNOGRAPHY SYSTEMS
17.4.2.3. NEUROLOGICAL MONITORING DEVICES
17.4.2.3.1. EEG MONITORS
17.4.2.3.2. ELECTROMYOGRAPHY (EMG) DEVICES
17.5 HEALTHCARE IT & DIGITAL HEALTH SOLUTIONS
17.5.1 TELEMEDICINE PLATFORMS
17.5.1.1. VIDEO CONSULTATION PLATFORMS
17.5.1.1.1. GENERAL TELECONSULTATION
17.5.1.1.2. TELEPSYCHIATRY
17.5.1.1.3. TELEDERMATOLOGY
17.5.1.1.4. TELENEUROLOGY
17.5.1.2. REMOTE PATIENT MANAGEMENT SYSTEMS
17.5.1.2.1. MOBILE HEALTH APPS
17.5.1.2.2. AI-BASED SYMPTOM CHECKERS
17.5.2 ELECTRONIC HEALTH RECORDS (EHR)
17.5.2.1. CLOUD-BASED EHR
17.5.2.2. ON-PREMISE EHR
17.5.2.3. AI-DRIVEN EHR SYSTEMS
17.5.3 AI-BASED DIAGNOSTIC & DECISION-SUPPORT TOOLS
17.5.3.1. AI IN RADIOLOGY & IMAGING
17.5.3.2. AI IN PATHOLOGY
17.5.3.3. AI FOR HISTOPATHOLOGICAL IMAGE ANALYSIS
17.5.3.4. AUTOMATED CYTOLOGY ANALYSIS
17.6 OTHERS
18. GLOBAL MEDICAL DEVICE ENGINEERING MARKET, BY APPLICATION
18.1 OVERVIEW
18.2 CARDIOVASCULAR
18.3 ORTHOPEDICS
18.4 NEUROLOGY
18.5 ONCOLOGY
18.6 RESPIRATORY
18.7 GENERAL SURGERY
18.8 OPHTHALMOLOGY
18.9 DENTAL
18.10 OTHERS
19. GLOBAL MEDICAL DEVICE ENGINEERING MARKET, BY END USER
19.1 OVERVIEW
19.2 MEDICAL DEVICE MANUFACTURERS
19.3 CONTRACT MANUFACTURING ORGANIZATIONS (CMOS) & CONTRACT DEVELOPMENT AND MANUFACTURING ORGANIZATIONS (CDMOS)
19.4 HOSPITALS & HEALTHCARE PROVIDERS
19.5 REMOTE PATIENT MONITORING PROVIDERS
19.6 REGULATORY & COMPLIANCE BODIES
19.7 OTHERS
20. GLOBAL MEDICAL DEVICE ENGINEERING MARKET, BY COUNTRY
GLOBAL MEDICAL DEVICE ENGINEERING MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
20.1 NORTH AMERICA
20.1.1 U.S.
20.1.2 CANADA
20.1.3 MEXICO
20.2 EUROPE
20.2.1 GERMANY
20.2.2 U.K.
20.2.3 ITALY
20.2.4 FRANCE
20.2.5 SPAIN
20.2.6 RUSSIA
20.2.7 SWITZERLAND
20.2.8 TURKEY
20.2.9 BELGIUM
20.2.10 NETHERLANDS
20.2.11 DENMARK
20.2.12 SWEDEN
20.2.13 POLAND
20.2.14 NORWAY
20.2.15 FINLAND
20.2.16 REST OF EUROPE
20.3 ASIA-PACIFIC
20.3.1 JAPAN
20.3.2 CHINA
20.3.3 SOUTH KOREA
20.3.4 INDIA
20.3.5 SINGAPORE
20.3.6 THAILAND
20.3.7 INDONESIA
20.3.8 MALAYSIA
20.3.9 PHILIPPINES
20.3.10 AUSTRALIA
20.3.11 NEW ZEALAND
20.3.12 VIETNAM
20.3.13 TAIWAN
20.3.14 REST OF ASIA-PACIFIC
20.4 SOUTH AMERICA
20.4.1 BRAZIL
20.4.2 ARGENTINA
20.4.3 REST OF SOUTH AMERICA
20.5 MIDDLE EAST AND AFRICA
20.5.1 SOUTH AFRICA
20.5.2 EGYPT
20.5.3 BAHRAIN
20.5.4 UNITED ARAB EMIRATES
20.5.5 KUWAIT
20.5.6 OMAN
20.5.7 QATAR
20.5.8 SAUDI ARABIA
20.5.9 REST OF MEA
20.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
21. GLOBAL MEDICAL DEVICE ENGINEERING MARKET, SWOT AND DBMR ANALYSIS
22. GLOBAL MEDICAL DEVICE ENGINEERING MARKET, COMPANY PROFILE
22.1 3M HEALTH CARE
22.1.1 COMPANY OVERVIEW
22.1.2 REVENUE ANALYSIS
22.1.3 GEOGRAPHIC PRESENCE
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 ABBOTT LABORATORIES
22.2.1 COMPANY OVERVIEW
22.2.2 REVENUE ANALYSIS
22.2.3 GEOGRAPHIC PRESENCE
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 BAXTER INTERNATIONAL
22.3.1 COMPANY OVERVIEW
22.3.2 REVENUE ANALYSIS
22.3.3 GEOGRAPHIC PRESENCE
22.3.4 PRODUCT PORTFOLIO
22.3.5 RECENT DEVELOPMENTS
22.4 BECTON DICKINSON COMPANY
22.4.1 COMPANY OVERVIEW
22.4.2 REVENUE ANALYSIS
22.4.3 GEOGRAPHIC PRESENCE
22.4.4 PRODUCT PORTFOLIO
22.4.5 RECENT DEVELOPMENTS
22.5 BOSTON SCIENTIFIC CORPORATION
22.5.1 COMPANY OVERVIEW
22.5.2 REVENUE ANALYSIS
22.5.3 GEOGRAPHIC PRESENCE
22.5.4 PRODUCT PORTFOLIO
22.5.5 RECENT DEVELOPMENTS
22.6 CANON MEDICAL SYSTEMS CORPORATION
22.6.1 COMPANY OVERVIEW
22.6.2 REVENUE ANALYSIS
22.6.3 GEOGRAPHIC PRESENCE
22.6.4 PRODUCT PORTFOLIO
22.6.5 RECENT DEVELOPMENTS
22.7 CARDINAL HEALTH
22.7.1 COMPANY OVERVIEW
22.7.2 REVENUE ANALYSIS
22.7.3 GEOGRAPHIC PRESENCE
22.7.4 PRODUCT PORTFOLIO
22.7.5 RECENT DEVELOPMENTS
22.8 DANAHER CORPORATION
22.8.1 COMPANY OVERVIEW
22.8.2 REVENUE ANALYSIS
22.8.3 GEOGRAPHIC PRESENCE
22.8.4 PRODUCT PORTFOLIO
22.8.5 RECENT DEVELOPMENTS
22.9 FRESENIUS MEDICAL CARE
22.9.1 COMPANY OVERVIEW
22.9.2 REVENUE ANALYSIS
22.9.3 GEOGRAPHIC PRESENCE
22.9.4 PRODUCT PORTFOLIO
22.9.5 RECENT DEVELOPMENTS
22.10 GE HEALTHCARE
22.10.1 COMPANY OVERVIEW
22.10.2 REVENUE ANALYSIS
22.10.3 GEOGRAPHIC PRESENCE
22.10.4 PRODUCT PORTFOLIO
22.10.5 RECENT DEVELOPMENTS
22.11 HOLOGIC, INC.
22.11.1 COMPANY OVERVIEW
22.11.2 REVENUE ANALYSIS
22.11.3 GEOGRAPHIC PRESENCE
22.11.4 PRODUCT PORTFOLIO
22.11.5 RECENT DEVELOPMENTS
22.12 INTUITIVE SURGICAL
22.12.1 COMPANY OVERVIEW
22.12.2 REVENUE ANALYSIS
22.12.3 GEOGRAPHIC PRESENCE
22.12.4 PRODUCT PORTFOLIO
22.12.5 RECENT DEVELOPMENTS
22.13 JOHNSON & JOHNSON
22.13.1 COMPANY OVERVIEW
22.13.2 REVENUE ANALYSIS
22.13.3 GEOGRAPHIC PRESENCE
22.13.4 PRODUCT PORTFOLIO
22.13.5 RECENT DEVELOPMENTS
22.14 MEDTRONIC PLC
22.14.1 COMPANY OVERVIEW
22.14.2 REVENUE ANALYSIS
22.14.3 GEOGRAPHIC PRESENCE
22.14.4 PRODUCT PORTFOLIO
22.14.5 RECENT DEVELOPMENTS
22.15 PHILIPS HEALTHCARE
22.15.1 COMPANY OVERVIEW
22.15.2 REVENUE ANALYSIS
22.15.3 GEOGRAPHIC PRESENCE
22.15.4 PRODUCT PORTFOLIO
22.15.5 RECENT DEVELOPMENTS
22.16 RESMED INC.
22.16.1 COMPANY OVERVIEW
22.16.2 REVENUE ANALYSIS
22.16.3 GEOGRAPHIC PRESENCE
22.16.4 PRODUCT PORTFOLIO
22.16.5 RECENT DEVELOPMENTS
22.17 ROCHE DIAGNOSTICS INTERNATIONAL LTD
22.17.1 COMPANY OVERVIEW
22.17.2 REVENUE ANALYSIS
22.17.3 GEOGRAPHIC PRESENCE
22.17.4 PRODUCT PORTFOLIO
22.17.5 RECENT DEVELOPMENTS
22.18 SIEMENS HEALTHINEERS AG
22.18.1 COMPANY OVERVIEW
22.18.2 REVENUE ANALYSIS
22.18.3 GEOGRAPHIC PRESENCE
22.18.4 PRODUCT PORTFOLIO
22.18.5 RECENT DEVELOPMENTS
22.19 SMITH & NEPHEW PLC.
22.19.1 COMPANY OVERVIEW
22.19.2 REVENUE ANALYSIS
22.19.3 GEOGRAPHIC PRESENCE
22.19.4 PRODUCT PORTFOLIO
22.19.5 RECENT DEVELOPMENTS
22.20 STRYKER CORPORATION
22.20.1 COMPANY OVERVIEW
22.20.2 REVENUE ANALYSIS
22.20.3 GEOGRAPHIC PRESENCE
22.20.4 PRODUCT PORTFOLIO
22.20.5 RECENT DEVELOPMENTS
22.21 TERUMO CORPORATION
22.21.1 COMPANY OVERVIEW
22.21.2 REVENUE ANALYSIS
22.21.3 GEOGRAPHIC PRESENCE
22.21.4 PRODUCT PORTFOLIO
22.21.5 RECENT DEVELOPMENTS
22.22 THERMO FISHER SCIENTIFIC, INC.
22.22.1 COMPANY OVERVIEW
22.22.2 REVENUE ANALYSIS
22.22.3 GEOGRAPHIC PRESENCE
22.22.4 PRODUCT PORTFOLIO
22.22.5 RECENT DEVELOPMENTS
22.23 ZIMMER BIOMET HOLDINGS, INC.
22.23.1 COMPANY OVERVIEW
22.23.2 REVENUE ANALYSIS
22.23.3 GEOGRAPHIC PRESENCE
22.23.4 PRODUCT PORTFOLIO
22.23.5 RECENT DEVELOPMENTS
22.24 FLEX HEALTH SOLUTIONS
22.24.1 COMPANY OVERVIEW
22.24.2 REVENUE ANALYSIS
22.24.3 GEOGRAPHIC PRESENCE
22.24.4 PRODUCT PORTFOLIO
22.24.5 RECENT DEVELOPMENTS
22.25 JABIL HEALTHCARE
22.25.1 COMPANY OVERVIEW
22.25.2 REVENUE ANALYSIS
22.25.3 GEOGRAPHIC PRESENCE
22.25.4 PRODUCT PORTFOLIO
22.25.5 RECENT DEVELOPMENTS
22.26 SANMINA CORPORATION
22.26.1 COMPANY OVERVIEW
22.26.2 REVENUE ANALYSIS
22.26.3 GEOGRAPHIC PRESENCE
22.26.4 PRODUCT PORTFOLIO
22.26.5 RECENT DEVELOPMENTS
22.27 CELESTICA HEALTHTECH
22.27.1 COMPANY OVERVIEW
22.27.2 REVENUE ANALYSIS
22.27.3 GEOGRAPHIC PRESENCE
22.27.4 PRODUCT PORTFOLIO
22.27.5 RECENT DEVELOPMENTS
22.28 INTEGER HOLDINGS CORPORATION
22.28.1 COMPANY OVERVIEW
22.28.2 REVENUE ANALYSIS
22.28.3 GEOGRAPHIC PRESENCE
22.28.4 PRODUCT PORTFOLIO
22.28.5 RECENT DEVELOPMENTS
RECENT DEVELOPMENTS NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST RELATED REPORTS
23. RELATED REPORTS
24. CONCLUSION
25. QUESTIONNAIRE
26. ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.