Global Medical Device Regulatory Affairs Outsourcing Market
Market Size in USD Billion
CAGR :
% 
 USD
7.36 Billion
USD
19.30 Billion
2024
2032
USD
7.36 Billion
USD
19.30 Billion
2024
2032
| 2025 –2032 | |
| USD 7.36 Billion | |
| USD 19.30 Billion | |
|
|
|
|
Medical Device Regulatory Affairs Outsourcing Market Size
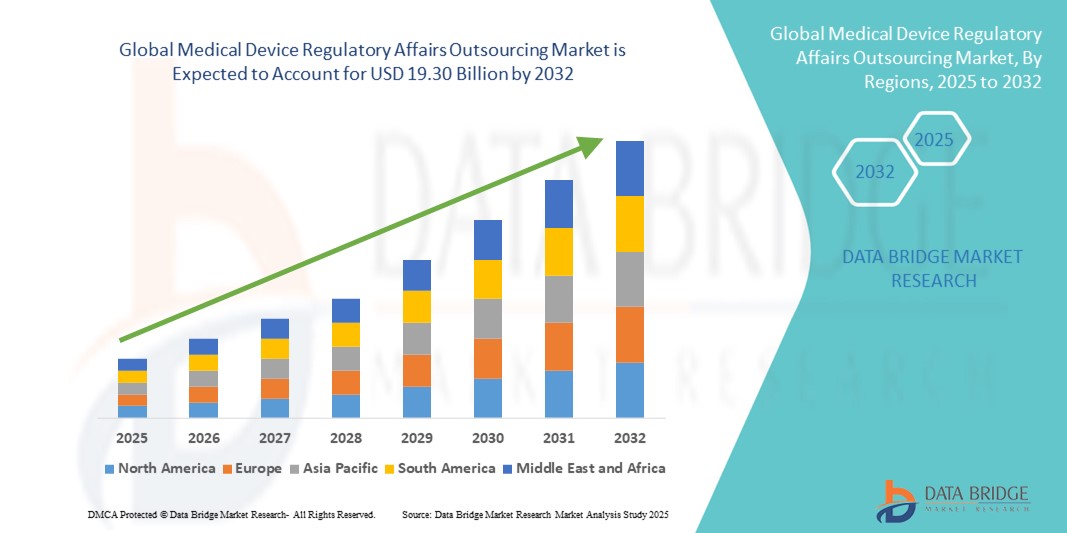
- The global medical device regulatory affairs outsourcing market size was valued at USD 7.36 billion in 2024 and is expected to reach USD 19.30 billion by 2032, at a CAGR of 12.8% during the forecast period
- The market growth is primarily driven by the increasing complexity of global regulatory requirements and the rising demand for faster product approvals, prompting medical device manufacturers to rely on specialized regulatory outsourcing services
- Additionally, growing R&D activities, frequent updates in compliance standards, and the pressure to reduce time-to-market are compelling companies to partner with external regulatory experts. These factors are accelerating the adoption of outsourcing solutions, significantly contributing to the market's expansion
Medical Device Regulatory Affairs Outsourcing Market Analysis
- Medical device regulatory affairs outsourcing involves delegating regulatory tasks such as product registration, compliance documentation, and liaison with authorities to specialized third-party firms, becoming a crucial strategy for manufacturers aiming to navigate increasingly complex global regulatory environments efficiently and cost-effectively
- The surging demand for regulatory outsourcing is primarily driven by the continuous updates in global compliance requirements, the need to accelerate market approvals, and the shortage of in-house regulatory expertise among small to mid-sized device manufacturers
- North America dominated the medical device regulatory affairs outsourcing market with the largest revenue share of 39.2% in 2024, supported by the region's mature medical device industry, strong regulatory framework, and high outsourcing penetration among U.S.-based manufacturers seeking faster FDA approvals
- Asia-Pacific is projected to witness the fastest growth in the market during the forecast period due to the region’s expanding medical device manufacturing base, evolving regulatory frameworks, and cost advantages offered by local CROs and regulatory consultancies
- The regulatory writing and publishing segment dominated the market with a share of 42% in 2024, attributed to its critical role in ensuring clear, compliant submissions to regulatory bodies, which directly impacts approval timelines and product launch success
Report Scope and Medical Device Regulatory Affairs Outsourcing Market Segmentation
|
Attributes |
Medical Device Regulatory Affairs Outsourcing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Medical Device Regulatory Affairs Outsourcing Market Trends
“Digital Transformation and AI-Powered Regulatory Solutions”
- A significant and accelerating trend in the global medical device regulatory affairs outsourcing market is the increasing adoption of digital technologies and artificial intelligence (AI) to enhance regulatory processes and compliance efficiency. This digital shift is streamlining operations and transforming how manufacturers approach complex, multi-country regulatory submissions
- For instance, regulatory service providers such as Freyr and IQVIA are deploying AI-enabled platforms for regulatory document automation, real-time submission tracking, and predictive regulatory intelligence, enabling faster and more accurate decision-making
- AI integration allows for automated compilation of technical files, identification of regulatory gaps, and proactive compliance with changing global standards. These capabilities significantly reduce time-to-market and resource burden for manufacturers
- The use of centralized digital platforms further enhances collaboration between outsourcing partners and internal regulatory teams, ensuring transparency, consistency, and ease of access to updated submission materials
- This trend toward smart regulatory solutions is reshaping expectations within the industry, with clients demanding faster turnaround, improved data accuracy, and scalable compliance support
- As the complexity of global regulations grows, the demand for intelligent, automated, and tech-driven regulatory outsourcing services is rising across both mature and emerging medical device markets, with companies increasingly viewing these partnerships as critical to their global expansion strategies
Medical Device Regulatory Affairs Outsourcing Market Dynamics
Driver
“Increasing Complexity of Global Regulatory Frameworks and Product Innovation”
- The growing intricacy of international regulatory standards and the continuous innovation in medical device technologies are key drivers accelerating the demand for regulatory affairs outsourcing services
- For instance, in January 2024, Parexel expanded its global regulatory consulting services to support companies navigating new EU MDR and IVDR frameworks, highlighting the increasing need for expert guidance in complex compliance environments
- Medical device manufacturers are under pressure to keep pace with evolving global requirements while ensuring timely market access, making external regulatory partners vital in maintaining both speed and accuracy
- The rise of digital health solutions, AI-enabled devices, and combination products has added further layers of regulatory scrutiny, compelling companies to rely on third-party experts for strategic submissions, ongoing compliance, and market surveillance
- By outsourcing these tasks, manufacturers can focus on innovation and commercialization while reducing internal resource burdens, accelerating approval timelines, and expanding into new markets more effectively
Restraint/Challenge
“Data Privacy Concerns and Talent Shortage”
- Rising concerns over data privacy and cybersecurity represent a major challenge in the global medical device regulatory affairs outsourcing market. As sensitive patient data and proprietary product information are transferred across borders, companies must ensure full compliance with data protection regulations such as GDPR and HIPAA
- For instance, any breach or mishandling of regulatory documentation by third-party providers could expose manufacturers to significant legal, financial, and reputational risks, potentially delaying product approvals or triggering regulatory penalties
- Additionally, the market faces a persistent shortage of skilled regulatory professionals with in-depth knowledge of region-specific device regulations and quality standards, leading to bottlenecks and quality control challenges for outsourcing firms
- While some firms are investing in workforce development and digital compliance infrastructure, bridging this talent and security gap is essential to gaining and retaining client trust
- Addressing these issues through enhanced data governance protocols, continuous upskilling, and strategic regional partnerships will be key to ensuring secure, efficient, and compliant regulatory affairs outsourcing in the long term
Medical Device Regulatory Affairs Outsourcing Market Scope
The market is segmented on the basis of services, product, device type, category, application, and end user.
- By Services
On the basis of services, the medical device regulatory affairs outsourcing market is segmented into regulatory affairs services, quality consulting, regulatory writing and publishing, regulatory submissions, clinical trial applications, product registrations, legal representation, and medical writing. The regulatory writing and publishing segment dominated the market with the largest market revenue share of 42% in 2024, driven by its essential role in the preparation and submission of compliant, detailed regulatory documentation across global agencies. Regulatory writing ensures clarity, accuracy, and consistency in submissions, which is critical for achieving timely product approvals and maintaining compliance throughout the product lifecycle.
The clinical trial applications segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by the increasing number of innovative devices entering clinical development. As regulatory frameworks become more stringent, outsourcing clinical application tasks to experienced regulatory service providers helps ensure accuracy and compliance, reducing delays in the approval process.
- By Product
On the basis of product, the medical device regulatory affairs outsourcing market is segmented into finished goods, electronics, and raw material. The finished goods segment held the largest market revenue share in 2024, attributed to the high volume of final medical devices requiring extensive documentation, testing, and regulatory approvals before commercialization. These products often involve comprehensive global submission strategies and require post-market surveillance support, driving demand for outsourcing.
The electronics segment is expected to witness the fastest CAGR from 2025 to 2032, driven by the increasing integration of digital technologies, AI, and software components in medical devices. Regulatory compliance for electronic components, especially in connected and wearable devices, requires specialized expertise in cybersecurity and technical file preparation, boosting outsourcing demand.
- By Device Type
On the basis of device type, the medical device regulatory affairs outsourcing market is segmented into Class I, Class II, and Class III. The Class II segment dominated the market with the largest market revenue share in 2024, due to the large number of moderately complex medical devices that fall under this classification. These devices require substantial regulatory documentation and quality system compliance, prompting manufacturers to seek external support for efficient and timely approvals.
The Class III segment is anticipated to witness the fastest growth rate from 2025 to 2032, as these high-risk devices demand extensive pre-market approvals, rigorous clinical trials, and long-term surveillance data. The complexity and risk level of these devices make outsourcing a strategic choice for manufacturers aiming to meet global regulatory expectations.
- By Category
On the basis of category, the medical device regulatory affairs outsourcing market is segmented into biologics, drugs, and medical devices. The medical devices segment dominated the market with the largest market revenue share in 2024, driven by the increasing innovation and volume of new devices being developed globally. The constant evolution of regulatory requirements specific to medical devices encourages manufacturers to outsource to specialized service providers for better compliance and efficiency.
The biologics segment is expected to witness the fastest growth from 2025 to 2032, fueled by the rise of combination products and personalized therapies. These products require multi-disciplinary regulatory approaches, making outsourcing essential for navigating complex approval pathways and ensuring coordinated compliance.
- By Application
On the basis of application, the medical device regulatory affairs outsourcing market is segmented into cardiology, diagnostic imaging, orthopedic, IVD, ophthalmic, general and plastic surgery, drug delivery, dental, endoscopy, diabetes care, and others. The IVD segment held the largest market revenue share in 2024, supported by the surge in diagnostic testing and the implementation of strict regulatory frameworks such as the EU IVDR. Regulatory outsourcing is critical for ensuring accurate classification, technical documentation, and performance evaluation data for these products.
The diabetes care segment is expected to witness the fastest CAGR during the forecast period, driven by the proliferation of innovative glucose monitoring devices and digital health solutions. These technologies require specialized regulatory strategies and continuous compliance monitoring, increasing reliance on outsourcing partners.
- By End User
On the basis of end user, the medical device regulatory affairs outsourcing market is segmented into small medical device company, medium medical device company, pharmaceutical and biotechnology companies, medical devices manufacturer, and large medical device company. The large medical device company segment dominated the market with the largest market revenue share in 2024, as these companies manage diverse portfolios across multiple global markets and rely on regulatory outsourcing to maintain operational efficiency and regulatory consistency.
The small medical device company segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by limited in-house regulatory resources and the need for expert guidance to meet complex compliance standards. These companies often outsource end-to-end regulatory tasks to accelerate market entry and reduce operational burdens.
Medical Device Regulatory Affairs Outsourcing Market Regional Analysis
- North America dominated the medical device regulatory affairs outsourcing market with the largest revenue share of 39.2% in 2024, supported by the region's mature medical device industry, strong regulatory framework, and high outsourcing penetration among U.S.-based manufacturers seeking faster FDA approvals
- Companies in the region prioritize timely product approvals and strategic global expansion, fueling reliance on outsourcing partners to manage complex submissions and evolving regulations such as the U.S. FDA's QMSR and EU MDR transition support
- This strong market position is further supported by a well-established ecosystem of regulatory consulting firms, robust R&D investment, and a high concentration of medical device manufacturers, making North America a key hub for outsourced regulatory services across all stages of product development and commercialization
U.S. Medical Device Regulatory Affairs Outsourcing Market Insight
The U.S. medical device regulatory affairs outsourcing market captured the largest revenue share of 84% in 2024 within North America, driven by the complexity of FDA regulatory processes and the increasing reliance on external expertise to expedite approvals. With a high volume of medical device innovation and growing pressure for faster market entry, U.S.-based manufacturers are outsourcing tasks such as submission preparation, regulatory strategy, and post-market compliance. Furthermore, the continued evolution of FDA regulations, including the QMSR update, is intensifying the need for specialized regulatory consulting services across all company sizes.
Europe Medical Device Regulatory Affairs Outsourcing Market Insight
The Europe medical device regulatory affairs outsourcing market is projected to expand at a substantial CAGR throughout the forecast period, primarily fueled by the implementation of the EU MDR and IVDR frameworks. These regulations have significantly increased documentation, clinical evidence, and surveillance requirements, driving demand for outsourcing partners with EU-specific regulatory expertise. The region’s strong base of small to mid-sized device manufacturers further contributes to outsourcing adoption, especially across key countries such as Germany, France, and the Netherlands, where timely CE marking is critical for commercial success.
U.K. Medical Device Regulatory Affairs Outsourcing Market Insight
The U.K. medical device regulatory affairs outsourcing market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by post-Brexit regulatory shifts and the growing need to navigate both UKCA and international compliance routes. Manufacturers in the U.K. are increasingly outsourcing regulatory tasks to ensure alignment with MHRA requirements while simultaneously pursuing global market access. The presence of highly specialized regulatory consultancies and a strong medtech ecosystem makes the U.K. a key contributor to regional market growth.
Germany Medical Device Regulatory Affairs Outsourcing Market Insight
The Germany medical device regulatory affairs outsourcing market is expected to expand at a considerable CAGR during the forecast period, driven by its position as a leading medical device manufacturing hub and a strict regulatory environment. As companies focus on meeting complex EU MDR compliance requirements, German manufacturers are turning to outsourcing firms for support in documentation, gap analysis, and clinical evaluations. The emphasis on precision, quality, and sustainability in German medtech is also encouraging collaboration with expert regulatory partners to maintain global competitiveness.
Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market Insight
The Asia-Pacific medical device regulatory affairs outsourcing market is poised to grow at the fastest CAGR of 25% during the forecast period of 2025 to 2032, driven by expanding medical device production, rising international exports, and increasingly sophisticated regulatory frameworks in countries such as China, Japan, and India. Regional governments are enhancing oversight and aligning with global standards, prompting local and global companies to outsource compliance tasks. Additionally, the cost-effectiveness and rapid scalability of outsourcing solutions in APAC are attracting multinational device manufacturers seeking regional approval and distribution.
Japan Medical Device Regulatory Affairs Outsourcing Market Insight
The Japan medical device regulatory affairs outsourcing market is gaining momentum due to its complex PMDA approval processes and the high rate of technological innovation within the country’s medtech sector. Japanese manufacturers are increasingly leveraging regulatory outsourcing for product classification, clinical trial support, and bilingual documentation to ensure timely local and international approvals. The demand for regulatory expertise in areas such as AI-enabled devices and combination products is further driving market growth in Japan.
India Medical Device Regulatory Affairs Outsourcing Market Insight
The India medical device regulatory affairs outsourcing market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to the country's expanding medical device manufacturing base, increasing compliance with CDSCO guidelines, and growing presence of domestic regulatory consultancies. The push for “Make in India” and smart healthcare infrastructure has led to an upsurge in product development, requiring efficient regulatory support. The availability of skilled professionals and cost advantages makes India a central hub for both local and international regulatory outsourcing.
Medical Device Regulatory Affairs Outsourcing Market Share
The medical device regulatory affairs outsourcing industry is primarily led by well-established companies, including:
- Parexel International (MA) Corporation (U.S.)
- North American Science Associates, LLC U.S.)
- SGS Société Générale de Surveillance SA. (Switzerland)
- Pace (U.S.)
- Trilogy Writing & Consulting GmbH (Germany)
- Creganna (Ireland)
- Intertek Group plc (U.K.)
- WuXi AppTec (China)
- Charles River Laboratories (U.S.)
- Celestica Inc. (Canada)
- Freyr (U.S.)
- Cactus Communications (India)
- In.Corp Indonesia (Indonesia)
- Eurofins Scientific (Luxembourg)
- Plexus Corp. (U.S.)
- Sanmina Corporation (U.S.)
- OMRON Corporation (Japan)
What are the Recent Developments in Global Medical Device Regulatory Affairs Outsourcing Market?
- In April 2023, Parexel International Corporation expanded its global regulatory consulting services to support medical device and combination product developers navigating the increasingly complex requirements under the European Union Medical Device Regulation (EU MDR) and In Vitro Diagnostic Regulation (IVDR). This strategic enhancement reflects Parexel’s commitment to helping clients accelerate market access by leveraging deep regulatory expertise and global presence, particularly amid tightening European compliance landscapes
- In March 2023, ICON plc announced the launch of a new Regulatory Intelligence platform designed to assist medical device companies in managing evolving global regulations and submission strategies. This digital solution provides real-time access to regulatory updates, compliance timelines, and document templates, enabling streamlined preparation and approval processes. The innovation underscores ICON’s focus on integrating technology with regulatory consulting to drive efficiency and accuracy for its clients
- In March 2023, Freyr Solutions opened a new global regulatory services hub in the Asia-Pacific region, aimed at supporting medical device companies with end-to-end compliance for country-specific approvals. The new center enhances Freyr’s capacity to manage growing demand from emerging markets and reinforces its position as a leader in regulatory affairs outsourcing by offering scalable, regionally tailored solutions
- In February 2023, IQVIA announced a strategic partnership with a European-based medical device manufacturer to support regulatory dossier preparation and clinical evaluation reporting under EU MDR. This collaboration emphasizes IQVIA’s strength in delivering comprehensive, customized regulatory services and reflects a broader trend of manufacturers relying on outsourcing to manage MDR complexities and mitigate risk
- In January 2023, Medistri SA, a Switzerland-based medtech service provider, introduced an integrated regulatory affairs and quality management consulting solution for small and mid-sized device manufacturers. This offering is designed to simplify the pathway to compliance for start-ups and emerging firms, providing cost-effective support for CE marking, technical file preparation, and post-market surveillance. The move demonstrates the industry's growing focus on scalable, accessible outsourcing services tailored to companies with limited in-house regulatory resources.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
- INTRODUCTION
- OBJECTIVES OF THE STUDY
- MARKET DEFINITION
- OVERVIEW OF GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET
- LIMITATIONS
- MARKETS COVERED
- MARKET SEGMENTATION
- MARKETS COVERED
- GEOGRAPHICAL SCOPE
- YEARS CONSIDERED FOR THE STUDY
- CURRENCY AND PRICING
- DBMR TRIPOD DATA VALIDATION MODEL
- MULTIVARIATE MODELLING
- SERVICES LIFELINE CURVE
- PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
- DBMR MARKET POSITION GRID
- VENDOR SHARE ANALYSIS
- MARKET APPLICATION COVERAGE GRID
- SECONDARY SOURCES
- ASSUMPTIONS
- EXECUTIVE SUMMARY
- PREMIUM INSIGHT
- MARKET OVERVIEW
- DRIVERS
- INCREASING GEOGRAPHICAL EXPANSION ACTIVITIES BY THE MEDICAL DEVICE COMPANIES
- INCREASING ADOPTION OF OUTSOURCING MODELS FOR REGULATORY SERVICES
- INCREASE IN R&D ACTIVITIES BY THE MEDICAL DEVICE COMPANIES
- RISING NUMBER OF CLINICAL TRIALS
- INCREASING NUMBER OF PATENT EXPIRATIONS
- RESTRAINTS
- FLUCTUATION IN PRICES
- HIGH COMPETITION AMONG KEY MARKET PLAYERS
- OPPORTUNITIES
- INCREASING SPENDING ON HEALTHCARE
- END-TO-END REGULATORY EXPERTISE
- AWARDS AND RECOGNITION
- CHALLENGES
- LACK OF ACCESSIBILITY
- PANDEMIC OUTBREAK OF COVID-19
- IMPACT OF COVID-19 ON THE GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET
- IMPACT ON PRICE
- IMPACT ON DEMAND
- IMPACT ON SUPPLY CHAIN
- STRATEGIC DECISIONS OF GOVERNMENT AND MANUFACTURERS
- CONCLUSION
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES
- OVERVIEW
- REGULATORY AFFAIRS SERVICES
- CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS
- CERTIFICATION SERVICES
- ISO 13485 AUDITS
- NOTIFIED BODY (EU)
- GLOBAL MARKET ACCESS
- CB SCHEME
- NRL & SCC (US & CAN)
- MDSAP
- REGULATORY WRITING AND PUBLISHING
- LEGAL REPRESENTATION
- OTHERS
- QUALITY CONSULTING
- MEDICAL WRITING
- CLINICAL WRITING
- REGULATORY WRITING
- SCIENTIFIC WRITING
- OTHERS
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY PRODUCT
- OVERVIEW
- FINISHED GOODS
- ELECTRONICS
- RAW MATERIALS
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEVICE TYPE
- OVERVIEW
- CLASS I
- CLASS II
- CLASS III
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY APPLICATION
- OVERVIEW
- CARDIOLOGY
- CLASS III
- CLASS I
- CLASS II
- GENERAL AND PLASTIC SURGERY
- CLASS I
- CLASS II
- CLASS III
- IVD
- CLASS I
- CLASS III
- CLASS II
- ORTHOPAEDIC
- CLASS III
- CLASS I
- CLASS II
- DIAGNOSTIC IMAGING
- CLASS II
- CLASS I
- CLASS III
- DENTAL
- CLASS I
- CLASS III
- CLASS II
- OPHTHALMIC
- CLASS I
- CLASS II
- CLASS III
- ENDOSCOPY
- CLASS II
- CLASS I
- CLASS III
- DIABETES CARE
- CLASS I
- CLASS II
- CLASS III
- DRUG DELIVERY
- CLASS II
- CLASS III
- CLASS I
- OTHERS
- CLASS I
- CLASS II
- CLASS III
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER
- OVERVIEW
- MEDIUM MEDICAL DEVICE COMPANY
- SMALL MEDICAL DEVICE COMPANY
- LARGE MEDICAL DEVICE COMPANY
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY GEOGRAPHY
- OVERVIEW
- NORTH AMERICA
- U.S.
- CANADA
- MEXICO
- EUROPE
- GERMANY
- FRANCE
- U.K.
- ITALY
- SPAIN
- RUSSIA
- TURKEY
- IRELAND
- BELGIUM
- NETHERLANDS
- SWITZERLAND
- REST OF EUROPE
- ASIA-PACIFIC
- JAPAN
- CHINA
- SOUTH KOREA
- INDIA
- AUSTRALIA
- SINGAPORE
- THAILAND
- MALAYSIA
- INDONESIA
- PHILIPPINES
- REST OF ASIA-PACIFIC
- SOUTH AMERICA
- BRAZIL
- ARGENTINA
- REST OF SOUTH AMERICA
- MIDDLE EAST AND AFRICA
- SOUTH AFRICA
- SAUDI ARABIA
- U.A.E.
- EGYPT
- ISRAEL
- REST OF MIDDLE EAST AND AFRICA
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY LANDSCAPE
- COMPANY SHARE ANALYSIS: GLOBAL
- COMPANY SHARE ANALYSIS: NORTH AMERICA
- COMPANY SHARE ANALYSIS: EUROPE
- COMPANY SHARE ANALYSIS: ASIA-PACIFIC
- SWOT ANALYSIS
- COMPANY PROFILE
- PLEXUS CORP.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- COMPANY SHARE ANLYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- CELESTICA INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- COMPANY SHARE ANLYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- FLEX LTD.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- COMPANY SHARE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- TE CONNECTIVITY
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- COMPANY SHARE ANLYSIS
- INDUSTRY & SOLUTION PORTFOLIO
- RECENT DEVELOPMENTS
- TECOMET, INC.
- COMPANY SNAPSHOT
- COMPANY SHARE ANLYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- PAREXEL INTERNATIONAL CORPORATION
- COMPANY SNAPSHOT
- SOLUTION PORTFOLIO
- RECENT DEVELOPMENTS
- SANMINA CORPORATION
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- INDUSTRY PORTFOLIO
- RECENT DEVELOPMENTS
- EUROFINS SCIENTIFIC
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENT
- AMERICAN PRECLINICAL SERVICES, LLC
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- CACTUS COMMUNICATIONS
- COMPANY SNAPSHOT
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- CEKINDO BUSINESS INTERNATIONAL
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- CHARLES RIVER LABORATORIES
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT & SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- COVANCE (A SUBSIDIARY OF LABORATORY CORPORATION OF AMERICA HOLDINGS)
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- CREGANNA (A SUBSIDIARY OF TE CONNECTIVITY)
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT & SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- EAST WEST MANUFACTURING
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- FREYR
- COMPANY SNAPSHOT
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- HERAEUS HOLDING
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- INTEGER HOLDINGS CORPORATION
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- INTERTEK GROUP PLC
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- IQVIA
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- NORTH AMERICAN SCIENCE ASSOCIATES, INC.
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- NORTECH SYSTEMS, INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- OMICS INTERNATIONAL
- COMPANY SNAPSHOT
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- OMRON CORPORATION
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- PACE ANALYTICAL SERVICES, LLC
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- JABIL INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- SGS SA
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- STERIGENICS U.S., LLC – A SOTERA HEALTH COMPANY
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- TRILOGY WRITING & CONSULTING GMBH
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- TÜV SÜD
- COMPANY SNAPSHOT
- SERVICE PORTFOLIO
- RECENT DEVELOPMENT
- WUXI APPTEC
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- QUESTIONNAIRE
- RELATED REPORTS
List of Table
TABLE 1 Global Medical device regulatory affairs outsourcing market, By services, 2019-2028 (USD million)
TABLE 2 Global Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD Million)
TABLE 3 Global Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 4 Global Clinical Trials Applications and Product Registrations IN Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 5 Global Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 6 Global Quality Consulting service in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD Million)
TABLE 7 Global Medical Writing service in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD Million)
TABLE 8 Global Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 9 Global Medical device regulatory affairs outsourcing market, By product, 2019-2028 (USD million)
TABLE 10 Global Finished Goods products in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 11 Global Electronics products in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 12 Global Raw Materials products in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 13 Global Medical device regulatory affairs outsourcing market, By device type, 2019-2028 (USD million)
TABLE 14 Global class i Device in Medical device regulatory affairs outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 15 Global Class II Device in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 16 Global Class III Device in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 17 Global Medical device regulatory affairs outsourcing market, By application, 2019-2028 (USD million)
TABLE 18 Global Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 19 Global Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 20 Global General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 21 Global General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 22 Global IVD in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 23 Global IVD in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 24 Global Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 25 Global Orthopedic in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 26 Global Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 27 Global Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 28 Global Dental in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 29 Global dental in Medical device regulatory affairs outsourcing market, By device type, 2019-2028 (USD million)
TABLE 30 Global Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 31 Global Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 32 Global Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 33 Global Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 34 Global Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 35 Global Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 36 Global Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 37 Global Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 38 Global Others in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 39 Global Others in Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD million)
TABLE 40 Global Medical device regulatory affairs outsourcing market, By end user, 2019-2028 (USD million)
TABLE 41 Global Medium Medical Device Company in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 42 Global Small Medical Device Company in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 43 Global Large Medical Device Company in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 44 GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 45 North America medical device regulatory affairs outsourcing Market, By COUNTRY, 2019-2028 (USD million)
TABLE 46 North America Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 47 North America Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 48 North America CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 49 North America CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 50 North America Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 51 North America Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 52 North America Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 53 North America Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 54 North America Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD Million)
TABLE 55 North America General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 56 North America IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 57 North America Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 58 North America Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 59 North America Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 60 North America Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 61 North America Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 62 North America Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 63 North America Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 64 North America Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 65 North America Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 66 U.S. Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 67 U.S. Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 68 U.S. CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 69 U.S. CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 70 U.S. Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 71 U.S. Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 72 U.S. Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 73 U.S. Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 74 U.S. Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 75 U.S. General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 76 U.S. IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 77 U.S. Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 78 U.S. Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 79 U.S. Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 80 U.S. Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 81 U.S. Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 82 U.S. Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 83 U.S. Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 84 U.S. Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 85 U.S. Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 86 CANADA Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 87 CANADA Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 88 CANADA CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 89 CANADA CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 90 CANADA Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 91 CANADA Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 92 CANADA Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 93 CANADA Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 94 CANADA Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 95 CANADA General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 96 CANADA IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 97 CANADA Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 98 CANADA Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 99 CANADA Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 100 CANADA Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 101 CANADA Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 102 CANADA Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 103 CANADA Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 104 CANADA Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 105 CANADA Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 106 MEXICO Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 107 MEXICO Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 108 MEXICO CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 109 MEXICO CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 110 MEXICO Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 111 MEXICO Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 112 MEXICO Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 113 MEXICO Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 114 MEXICO Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 115 MEXICO General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 116 MEXICO IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 117 MEXICO Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 118 MEXICO Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 119 MEXICO Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 120 MEXICO Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 121 MEXICO Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 122 MEXICO Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 123 MEXICO Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 124 MEXICO Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 125 MEXICO Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 126 Europe medical device regulatory affairs outsourcing Market, By COUNTRY, 2021-2028 (USD million)
TABLE 127 Europe Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 128 Europe Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 129 Europe CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 130 Europe CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 131 Europe Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 132 Europe Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 133 Europe Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 134 Europe Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 135 Europe Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 136 Europe General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 137 Europe IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 138 Europe Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 139 Europe Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 140 Europe Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 141 Europe Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 142 Europe Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 143 Europe Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 144 Europe Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 145 Europe Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 146 Europe Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 147 Germany Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 148 Germany Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 149 Germany CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 150 Germany CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 151 Germany Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 152 Germany Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 153 Germany Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 154 Germany Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 155 Germany Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 156 Germany General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 157 Germany IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 158 Germany Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 159 Germany Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 160 Germany Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 161 Germany Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 162 Germany Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 163 Germany Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 164 Germany Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 165 Germany Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 166 Germany Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 167 France Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 168 France Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 169 France CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 170 France CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 171 France Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 172 France Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 173 France Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 174 France Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 175 France Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 176 France General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 177 France IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 178 France Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 179 France Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 180 France Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 181 France Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 182 France Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 183 France Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 184 France Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 185 France Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 186 France Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 187 U.K. Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 188 U.K. Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 189 U.K. CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 190 U.K. CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 191 U.K. Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 192 U.K. Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 193 U.K. Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 194 U.K. Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 195 U.K. Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 196 U.K. General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 197 U.K. IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 198 U.K. Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 199 U.K. Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 200 U.K. Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 201 U.K. Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 202 U.K. Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 203 U.K. Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 204 U.K. Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 205 U.K. Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 206 U.K. Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 207 Italy Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 208 Italy Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 209 Italy CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 210 Italy CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 211 Italy Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 212 Italy Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 213 Italy Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 214 Italy Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 215 Italy Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 216 Italy General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 217 Italy IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 218 Italy Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 219 Italy Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 220 Italy Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 221 Italy Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 222 Italy Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 223 Italy Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 224 Italy Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 225 Italy Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 226 Italy Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 227 Spain Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 228 Spain Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 229 Spain CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 230 Spain CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 231 Spain Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 232 Spain Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 233 Spain Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 234 Spain Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 235 Spain Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 236 Spain General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 237 Spain IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 238 Spain Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 239 Spain Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 240 Spain Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 241 Spain Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 242 Spain Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 243 Spain Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 244 Spain Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 245 Spain Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 246 Spain Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 247 Russia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 248 Russia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 249 Russia CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 250 Russia CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 251 Russia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 252 Russia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 253 Russia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 254 Russia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 255 Russia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 256 Russia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 257 Russia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 258 Russia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 259 Russia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 260 Russia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 261 Russia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 262 Russia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 263 Russia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 264 Russia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 265 Russia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 266 Russia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 267 Turkey Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 268 Turkey Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 269 Turkey CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 270 Turkey CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 271 Turkey Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 272 Turkey Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 273 Turkey Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 274 Turkey Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 275 Turkey Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 276 Turkey General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 277 Turkey IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 278 Turkey Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 279 Turkey Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 280 Turkey Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 281 Turkey Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 282 Turkey Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 283 Turkey Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 284 Turkey Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 285 Turkey Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 286 Turkey Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 287 Ireland Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 288 Ireland Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 289 Ireland CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 290 Ireland CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 291 Ireland Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 292 Ireland Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 293 Ireland Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 294 Ireland Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 295 Ireland Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 296 Ireland General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 297 Ireland IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 298 Ireland Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 299 Ireland Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 300 Ireland Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 301 Ireland Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 302 Ireland Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 303 Ireland Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 304 Ireland Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 305 Ireland Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 306 Ireland Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 307 Belgium Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 308 Belgium Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 309 Belgium CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 310 Belgium CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 311 Belgium Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 312 Belgium Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 313 Belgium Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 314 Belgium Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 315 Belgium Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 316 Belgium General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 317 Belgium IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 318 Belgium Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 319 Belgium Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 320 Belgium Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 321 Belgium Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 322 Belgium Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 323 Belgium Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 324 Belgium Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 325 Belgium Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 326 Belgium Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 327 Netherlands Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 328 Netherlands Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 329 Netherlands CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 330 Netherlands CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 331 Netherlands Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 332 Netherlands Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 333 Netherlands Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 334 Netherlands Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 335 Netherlands Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 336 Netherlands General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 337 Netherlands IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 338 Netherlands Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 339 Netherlands Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 340 Netherlands Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 341 Netherlands Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 342 Netherlands Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 343 Netherlands Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 344 Netherlands Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 345 Netherlands Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 346 Netherlands Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 347 Switzerland Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 348 Switzerland Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 349 Switzerland CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 350 Switzerland CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 351 Switzerland Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 352 Switzerland Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 353 Switzerland Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 354 Switzerland Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 355 Switzerland Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 356 Switzerland General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 357 Switzerland IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 358 Switzerland Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 359 Switzerland Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 360 Switzerland Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 361 Switzerland Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 362 Switzerland Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 363 Switzerland Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 364 Switzerland Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 365 Switzerland Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 366 Switzerland Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 367 REST OF EUROPE Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 368 Asia-Pacific medical device regulatory affairs outsourcing Market, By COUNTRY, 2019-2028 (USD MILLION)
TABLE 369 Asia-Pacific Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 370 Asia-Pacific Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 371 Asia-Pacific Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 372 Asia-Pacific Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 373 Asia-Pacific Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 374 Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 375 Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 376 Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 377 Asia-Pacific Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD Million)
TABLE 378 Asia-Pacific General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 379 Asia-Pacific IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 380 Asia-Pacific Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 381 Asia-Pacific Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 382 Asia-Pacific Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 383 Asia-Pacific Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 384 Asia-Pacific Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 385 Asia-Pacific Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 386 Asia-Pacific Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 387 Asia-Pacific Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 388 Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 389 Japan Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 390 Japan Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 391 Japan Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 392 Japan Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 393 Japan Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 394 Japan Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 395 Japan Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 396 Japan Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 397 Japan Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 398 Japan General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 399 Japan IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 400 Japan Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 401 Japan Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 402 Japan Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 403 Japan Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 404 Japan Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 405 Japan Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 406 Japan Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 407 Japan Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 408 Japan Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 409 China Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 410 China Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 411 China Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 412 China Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 413 China Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 414 China Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 415 China Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 416 China Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 417 China Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 418 China General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 419 China IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 420 China Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 421 China Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 422 China Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 423 China Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 424 China Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 425 China Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 426 China Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 427 China Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 428 China Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 429 South Korea Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 430 South Korea Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 431 South Korea Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 432 South Korea Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 433 South Korea Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 434 South Korea Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 435 South Korea Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 436 South Korea Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 437 South Korea Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 438 South Korea General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 439 South Korea IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 440 South Korea Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 441 South Korea Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 442 South Korea Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 443 South Korea Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 444 South Korea Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 445 South Korea Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 446 South Korea Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 447 South Korea Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 448 South Korea Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 449 India Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 450 India Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 451 India Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 452 India Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 453 India Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 454 India Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 455 India Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 456 India Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 457 India Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 458 India General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 459 India IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 460 India Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 461 India Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 462 India Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 463 India Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 464 India Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 465 India Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 466 India Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 467 India Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 468 India Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 469 Australia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 470 Australia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 471 Australia Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 472 Australia Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 473 Australia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 474 Australia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 475 Australia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 476 Australia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 477 Australia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 478 Australia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 479 Australia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 480 Australia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 481 Australia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 482 Australia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 483 Australia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 484 Australia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 485 Australia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 486 Australia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 487 Australia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 488 Australia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 489 Singapore Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 490 Singapore Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 491 Singapore Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 492 Singapore Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 493 Singapore Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 494 Singapore Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 495 Singapore Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 496 Singapore Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 497 Singapore Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 498 Singapore General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 499 Singapore IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 500 Singapore Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 501 Singapore Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 502 Singapore Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 503 Singapore Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 504 Singapore Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 505 Singapore Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 506 Singapore Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 507 Singapore Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 508 Singapore Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 509 Thailand Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 510 Thailand Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 511 Thailand Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 512 Thailand Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 513 Thailand Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 514 Thailand Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 515 Thailand Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 516 Thailand Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 517 Thailand Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 518 Thailand General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 519 Thailand IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 520 Thailand Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 521 Thailand Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 522 Thailand Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 523 Thailand Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 524 Thailand Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 525 Thailand Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 526 Thailand Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 527 Thailand Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 528 Thailand Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 529 Malaysia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 530 Malaysia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 531 Malaysia Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 532 Malaysia Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 533 Malaysia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 534 Malaysia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 535 Malaysia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 536 Malaysia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 537 Malaysia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 538 Malaysia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 539 Malaysia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 540 Malaysia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 541 Malaysia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 542 Malaysia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 543 Malaysia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 544 Malaysia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 545 Malaysia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 546 Malaysia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 547 Malaysia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 548 Malaysia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 549 Indonesia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 550 Indonesia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 551 Indonesia Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 552 Indonesia Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 553 Indonesia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 554 Indonesia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 555 Indonesia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 556 Indonesia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 557 Indonesia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 558 Indonesia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 559 Indonesia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 560 Indonesia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 561 Indonesia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 562 Indonesia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 563 Indonesia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 564 Indonesia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 565 Indonesia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 566 Indonesia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 567 Indonesia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 568 Indonesia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 569 Philippines Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 570 Philippines Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 571 Philippines Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 572 Philippines Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 573 Philippines Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 574 Philippines Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 575 Philippines Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 576 Philippines Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 577 Philippines Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 578 Philippines General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 579 Philippines IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 580 Philippines Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 581 Philippines Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 582 Philippines Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 583 Philippines Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 584 Philippines Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 585 Philippines Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 586 Philippines Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 587 Philippines Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 588 Philippines Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 589 Rest of Asia-Pacific Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 590 South America medical device regulatory affairs outsourcing Market, By COUNTRY, 2019-2028 (USD million)
TABLE 591 South America Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 592 South America Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 593 South America Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 594 South America Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 595 South America Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 596 South America Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 597 South America Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 598 South America Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 599 South America Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 600 South America General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 601 South America IVD in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 602 South America Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 603 South America Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 604 South America Dental in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 605 South America Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 606 South America Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 607 South America Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 608 South America Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 609 South America Others in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 610 South America Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 611 Brazil Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 612 Brazil Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 613 Brazil Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 614 Brazil Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 615 Brazil Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 616 Brazil Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 617 Brazil Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 618 Brazil Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 619 Brazil Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 620 Brazil General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 621 Brazil IVD in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 622 Brazil Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 623 Brazil Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 624 Brazil Dental in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 625 Brazil Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 626 Brazil Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 627 Brazil Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 628 Brazil Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 629 Brazil Others in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 630 Brazil Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 631 Argentina Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 632 Argentina Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 633 Argentina Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 634 Argentina Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 635 Argentina Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 636 Argentina Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 637 Argentina Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 638 Argentina Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 639 Argentina Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 640 Argentina General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 641 Argentina IVD in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 642 Argentina Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 643 Argentina Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 644 Argentina Dental in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 645 Argentina Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 646 Argentina Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 647 Argentina Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 648 Argentina Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 649 Argentina Others in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 650 Argentina Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 651 Rest of South America Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 652 Middle East and Africa medical device regulatory affairs outsourcing Market, By COUNTRY, 2019-2028 (USD million)
TABLE 653 Middle East and Africa Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 654 Middle East and Africa Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 655 Middle East and Africa Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 656 Middle East and Africa Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 657 Middle East and Africa Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 658 Middle East and Africa Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 659 Middle East and Africa Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 660 Middle East and Africa Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 661 Middle East and Africa Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 662 Middle East and Africa General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 663 Middle East and Africa IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 664 Middle East and Africa Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 665 Middle East and Africa Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 666 Middle East and Africa Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 667 Middle East and Africa Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 668 Middle East and Africa Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 669 Middle East and Africa Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 670 Middle East and Africa Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 671 Middle East and Africa Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 672 Middle East and Africa Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 673 South Africa Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 674 South Africa Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 675 South Africa Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 676 South Africa Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 677 South Africa Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 678 South Africa Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 679 South Africa Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 680 South Africa Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 681 South Africa Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 682 South Africa General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 683 South Africa IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 684 South Africa Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 685 South Africa Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 686 South Africa Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 687 South Africa Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 688 South Africa Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 689 South Africa Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 690 South Africa Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 691 South Africa Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 692 South Africa Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 693 Saudi Arabia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 694 Saudi Arabia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 695 Saudi Arabia Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 696 Saudi Arabia Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 697 Saudi Arabia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 698 Saudi Arabia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 699 Saudi Arabia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 700 Saudi Arabia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 701 Saudi Arabia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 702 Saudi Arabia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 703 Saudi Arabia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 704 Saudi Arabia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 705 Saudi Arabia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 706 audi Arabia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 707 Saudi Arabia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 708 Saudi Arabia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 709 Saudi Arabia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 710 Saudi Arabia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 711 Saudi Arabia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 712 Saudi Arabia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 713 U.A.E. Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 714 U.A.E. Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 715 U.A.E. Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 716 U.A.E. Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 717 U.A.E. Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 718 U.A.E. Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 719 U.A.E. Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 720 U.A.E. Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 721 U.A.E. Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 722 U.A.E. General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 723 U.A.E. IVD in Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 724 U.A.E. Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 725 U.A.E. Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 726 U.A.E. Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 727 U.A.E. Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 728 U.A.E. Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 729 U.A.E. Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 730 U.A.E. Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 731 U.A.E. Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 732 U.A.E. Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 733 Egypt Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 734 Egypt Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 735 Egypt Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 736 Egypt Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 737 Egypt Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 738 Egypt Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 739 Egypt Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 740 Egypt Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 741 Egypt Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 742 Egypt General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 743 Egypt IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 744 Egypt Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 745 Egypt Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 746 Egypt Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 747 Egypt Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 748 Egypt Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 749 Egypt Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 750 Egypt Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 751 Egypt Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 752 Egypt Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 753 Israel Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 754 Israel Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 755 Israel Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 756 Israel Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 757 Israel Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 758 Israel Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 759 Israel Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 760 Israel Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 761 Israel Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 762 Israel General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 763 Israel IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 764 Israel Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 765 Israel Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 766 Israel Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 767 Israel Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 768 Israel Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 769 Israel Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 770 Israel Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 771 Israel Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 772 Israel Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 773 Rest of Middle East and Africa Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
List of Figure
FIGURE 1 Global MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: SEGMENTATION
FIGURE 2 GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: DATA TRIANGULATION
FIGURE 3 global Medical device regulatory affairs outsourcing market: DROC ANALYSIS
FIGURE 4 Global Medical device regulatory affairs outsourcing market: Global VS. REGIONAL MARKET ANALYSIS
FIGURE 5 Global Medical device regulatory affairs outsourcing market: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL Medical device regulatory affairs outsourcing market: MULTIVARIATE MODELLING
FIGURE 7 GLOBAL Medical device regulatory affairs outsourcing market: INTERVIEW DEMOGRAPHICS
FIGURE 8 GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL medical device regulatory affairs outsourcing market: MARKET application COVERAGE GRID
FIGURE 11 Global Medical device regulatory affairs outsourcing market: SEGMENTATION
FIGURE 12 NORTH AMERICA is expected to DOMINATE THE GLOBAL Medical device regulatory affairs outsourcing market, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 13 Increasing adoption of outsourcing models for regulatory services is DRIVing THE global Medical device regulatory affairs outsourcing market IN THE FORECAST PERIOD OF 2021 to 2028
FIGURE 14 Regulatory Affairs Services SEGMENT is expected to account for the largest share of the global MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET in 2021 & 2028
FIGURE 15 ASIA-PACIFIC is the fastest growing market FOR the Medical device regulatory affairs outsourcing MANUFACTURERS IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGEs OF global Medical Device Regulatory Affairs Outsourcing Market
FIGURE 17 Global Medical device regulatory affairs outsourcing market: By services, 2020
FIGURE 18 Global Medical device regulatory affairs outsourcing market: By services, 2021-2028 (USD Million)
FIGURE 19 Global Medical device regulatory affairs outsourcing market: By services, CAGR (2021-2028)
FIGURE 20 Global Medical device regulatory affairs outsourcing market: By services, LIFELINE CURVE
FIGURE 21 Global Medical device regulatory affairs outsourcing market: By product, 2020
FIGURE 22 Global Medical device regulatory affairs outsourcing market: By product, 2021-2028 (USD million)
FIGURE 23 Global Medical device regulatory affairs outsourcing market: By product, CAGR (2021-2028)
FIGURE 24 Global Medical device regulatory affairs outsourcing market: By product, LIFELINE CURVE
FIGURE 25 Global Medical device regulatory affairs outsourcing market: By device type, 2020
FIGURE 26 Global Medical device regulatory affairs outsourcing market: By device type, 2021-2028 (USD million)
FIGURE 27 Global Medical device regulatory affairs outsourcing market: By device type, CAGR (2021-2028)
FIGURE 28 Global Medical device regulatory affairs outsourcing market: By device type, LIFELINE CURVE
FIGURE 29 Global Medical device regulatory affairs outsourcing market: By application, 2020
FIGURE 30 Global Medical device regulatory affairs outsourcing market: By application, 2021-2028 (USD million)
FIGURE 31 Global Medical device regulatory affairs outsourcing market: By application, CAGR (2021-2028)
FIGURE 32 Global Medical device regulatory affairs outsourcing market: By application, LIFELINE CURVE
FIGURE 33 Global Medical device regulatory affairs outsourcing market: By end user, 2020
FIGURE 34 Global Medical device regulatory affairs outsourcing market: By end user, 2021-2028 (USD million)
FIGURE 35 Global Medical device regulatory affairs outsourcing market: By end user, CAGR (2021-2028)
FIGURE 36 Global Medical device regulatory affairs outsourcing market: By end user, LIFELINE CURVE
FIGURE 37 GLobal medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 38 global medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 39 global medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2022 & 2028)
FIGURE 40 global medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 41 global medical device regulatory affairs outsourcing MARKET: BY SERVICES (2021-2028)
FIGURE 42 North America medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 43 North America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 44 North America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 45 North America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 46 North America medical device regulatory affairs outsourcing MARKET: BY SERVICES (2021-2028)
FIGURE 47 Europe medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 48 Europe medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 49 Europe medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 50 Europe medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 51 Europe medical device regulatory affairs outsourcing MARKET: BY SERVICES (2021-2028)
FIGURE 52 Asia-Pacific medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 53 Asia-Pacific medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 54 Asia-Pacific medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 55 Asia-Pacific medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 56 Asia-Pacific medical device regulatory affairs outsourcing MARKET: BY SERVICES (2021-2028)
FIGURE 57 South America medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 58 South America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 59 South America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 60 South America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 61 South America medical device regulatory affairs outsourcing MARKET: BY services (2021-2028)
FIGURE 62 Middle East and Africa medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 63 Middle East and Africa medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 64 Middle East and Africa medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 65 Middle East and Africa medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 66 Middle East and Africa medical device regulatory affairs outsourcing MARKET: BY Services (2021-2028)
FIGURE 67 Global Medical device regulatory affairs outsourcing Market: company share 2020 (%)
FIGURE 68 North America Medical device regulatory affairs outsourcing Market: company share 2020 (%)
FIGURE 69 Europe Medical device regulatory affairs outsourcing Market: company share 2020 (%)
FIGURE 70 Asia-Pacific Medical device regulatory affairs outsourcing Market: company share 2020 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.