Global Megalencephaly Polymicrogyria Polydactyly Hydrocephalus Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
60.50 Million
USD
86.69 Million
2024
2032
USD
60.50 Million
USD
86.69 Million
2024
2032
| 2025 –2032 | |
| USD 60.50 Million | |
| USD 86.69 Million | |
|
|
|
|
Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market Size
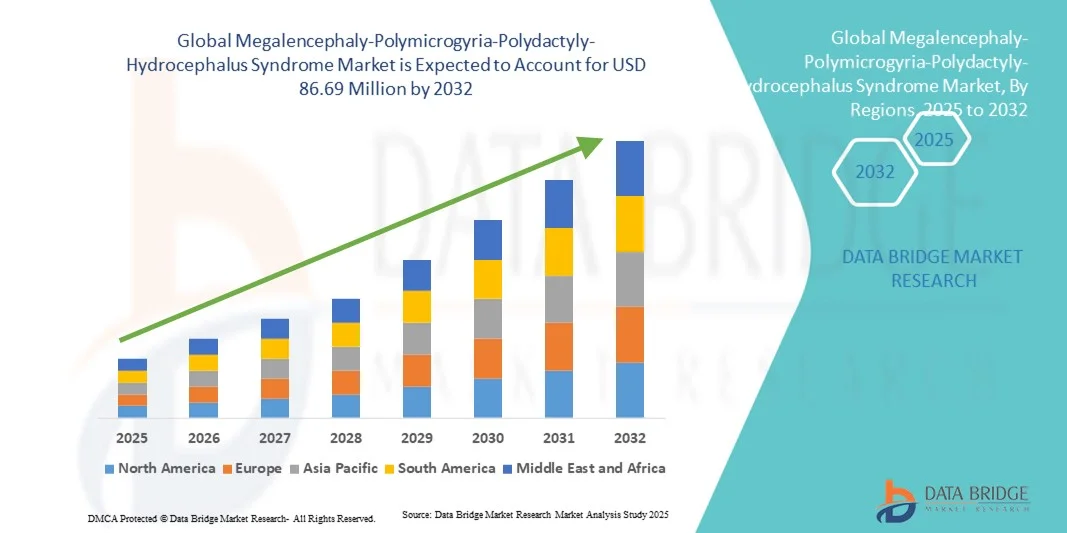
- The global Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome market size was valued at USD 60.50 million in 2024 and is expected to reach USD 86.69 million by 2032, at a CAGR of 4.60% during the forecast period
- The market growth is largely fueled by advances in genomic sequencing, molecular diagnostics, and precision medicine approaches that have improved early detection and classification of ultra-rare neurological syndromes such as MPPH
- Furthermore, growing investment by biotechnology and pharmaceutical companies in orphan drug development, coupled with supportive government frameworks and funding for rare neurological disorders, is positioning MPPH-focused research as an emerging niche within the rare disease therapeutics landscape. These converging factors are accelerating innovation, collaborative clinical research, and access to targeted therapies, thereby significantly boosting the industry’s growth
Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market Analysis
- Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus (MPPH) Syndrome is a rare congenital neurodevelopmental disorder involving abnormal brain growth and cortical development, necessitating multidisciplinary diagnosis and care approaches across neurology, genetics, and pediatrics for effective disease management
- The escalating demand in the MPPH Syndrome market is primarily fueled by advancements in genomic sequencing, improved neuroimaging diagnostics, and rising awareness of rare genetic disorders. Increasing research collaborations targeting PI3K-AKT-MTOR pathway mutations are further propelling the development of precision therapies and genetic counseling service
- North America dominated the MPPH Syndrome market with the largest revenue share of 41.8% in 2024, supported by strong rare-disease research networks, availability of advanced diagnostic infrastructure, and the presence of major academic and healthcare institutions focusing on early diagnosis and treatment innovation
- Asia-Pacific is expected to be the fastest-growing region in the MPPH Syndrome market during the forecast period due to expanding healthcare access, growing genomic testing capabilities, and government initiatives promoting rare-disease registries and clinical trials across China, Japan, and India
- The Drugs segment dominated the MPPH Syndrome market with a market share of 45.9% in 2024, driven by increasing use of anti-epileptic and neuroprotective agents to manage associated symptoms such as seizures and intellectual disabilities, alongside supportive therapies aimed at improving neurological outcomes and quality of life
Report Scope and Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market Segmentation
|
Attributes |
Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market Trends
Advancements in Genomic Medicine and Personalized Therapeutics
- A significant and accelerating trend in the global MPPH Syndrome market is the growing application of genomic sequencing, bioinformatics, and precision medicine approaches to better understand and manage this ultra-rare neurodevelopmental disorder, leading to more accurate diagnoses and personalized treatment strategies
- For instance, in 2024, researchers at the University of Cambridge utilized whole-exome sequencing to identify new pathogenic variants in AKT3 and PIK3R2 genes, improving genotype-phenotype correlations and enabling targeted therapeutic research
- Integration of AI and machine learning in genomic data analysis allows clinicians to predict clinical severity, design individualized care plans, and explore potential drug targets for related PI3K-AKT-MTOR pathway abnormalities
- Furthermore, AI-driven clinical decision support systems and genetic counseling platforms are enhancing diagnostic yield and family risk assessment, promoting earlier intervention and better care coordination for affected individuals
- The convergence of genomic innovation, digital health platforms, and translational research is fundamentally reshaping how rare neurological conditions such as MPPH are studied and managed, shifting the focus from symptom-based care to precision-based therapies
- This trend toward personalized and molecularly guided treatment is rapidly gaining traction across global research institutions and biotech firms, fostering a more collaborative ecosystem focused on discovery and clinical advancement in rare genetic syndromes
Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market Dynamics
Driver
Increasing Genetic Research and Expanding Rare Disease Infrastructure
- The growing emphasis on genetic research, rare disease registries, and precision diagnostics is a major driver for the MPPH Syndrome market, enabling early identification and improved disease understanding
- For instance, in March 2024, the National Institutes of Health (NIH) expanded its Rare Diseases Clinical Research Network (RDCRN) to include neurogenetic disorders such as MPPH, promoting data sharing and global collaboration
- As awareness of genetic and neurological disorders increases, healthcare systems are investing in advanced molecular testing and bioinformatics platforms to facilitate faster diagnosis and patient stratification
- Furthermore, international collaborations between academic institutions, hospitals, and biotech companies are accelerating translational research focused on pathway-specific drug development and gene-based interventions
- The growing adoption of next-generation sequencing in clinical settings, combined with supportive government initiatives for orphan drug research, is driving market expansion and innovation in MPPH management
- The increased recognition of ultra-rare disorders by healthcare policymakers and funding agencies is further stimulating research investment and fostering innovation in therapeutic development for underserved patient populations
Restraint/Challenge
Limited Clinical Data and High Research & Development Costs
- The extreme rarity of MPPH Syndrome poses a major challenge, as the limited number of diagnosed patients restricts the availability of large-scale clinical data necessary for robust drug and therapy development
- For instance, most current studies on MPPH involve fewer than 50 documented cases globally, making it difficult to establish standardized treatment protocols or conduct statistically significant clinical trials
- This scarcity of patient data also hampers the ability of researchers to perform long-term follow-up studies, leading to slower therapeutic validation and regulatory approvals
- In addition, the high cost of genetic testing, personalized therapy design, and advanced neuroimaging continues to be a major barrier to access, particularly in low- and middle-income regions
- Pharmaceutical and biotech companies face significant financial risks when developing therapies for ultra-rare diseases due to limited market size and uncertain reimbursement frameworks
- Overcoming these challenges through data-sharing collaborations, public-private partnerships, and funding incentives for rare-disease innovation will be critical to ensuring long-term progress in the MPPH Syndrome market
Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market Scope
The market is segmented on the basis of symptoms, treatment, route of administration, end-users, and distribution channel.
- By Symptoms
On the basis of symptoms, the MPPH Syndrome market is segmented into polymicrogyria, megalencephaly, intellectual disability, seizures, polydactyly, and hydrocephalus. The Polymicrogyria segment dominated the market with the largest revenue share in 2024, owing to its high prevalence among diagnosed MPPH cases and its critical role in neurological dysfunction and developmental delay. Clinically, polymicrogyria often represents the earliest detectable structural brain anomaly, leading to increased reliance on neuroimaging and genetic testing for early identification. The segment benefits from advances in MRI technology and neurodevelopmental research that aid in better characterization and management. Growing diagnostic awareness among clinicians and parents has further contributed to segment growth. The demand for multidisciplinary interventions, including speech and physical therapies, continues to rise as more patients are identified through enhanced genomic programs.
The Seizures segment is anticipated to witness the fastest growth from 2025 to 2032, driven by increasing recognition of epilepsy as a major comorbidity in MPPH Syndrome. The segment’s growth is supported by expanding use of anti-epileptic drugs and the emergence of targeted neuromodulatory treatments to control severe seizure episodes. For instance, recent studies emphasize pathway-based therapy approaches to manage seizure frequency in patients with PI3K-AKT-MTOR mutations. The growing focus on seizure control to improve quality of life and prevent neurological deterioration is propelling this segment’s rapid expansion across clinical and hospital settings.
- By Treatment
On the basis of treatment, the market is segmented into neurosurgery, genetic therapy, drugs, and others. The Drugs segment dominated the market in 2024 with the largest revenue share of 45.9%, supported by the widespread use of anti-epileptic, neuroprotective, and anti-inflammatory medications in managing symptoms associated with MPPH Syndrome. Pharmaceutical interventions remain the first line of care due to the absence of curative therapies, emphasizing symptomatic control and prevention of complications. Increasing availability of tailored drug regimens and ongoing research into repurposing existing neurological medications are bolstering this segment’s growth. In addition, rising hospital-based prescriptions and better access to supportive pharmacological therapies are enhancing treatment outcomes and segment dominance.
The Genetic Therapy segment is projected to witness the fastest growth rate during the forecast period, driven by increasing global R&D investments in gene-targeted and molecular therapies for PI3K-AKT-MTOR pathway-related disorders. For instance, researchers are exploring the use of precision CRISPR-Cas9 technologies and mTOR inhibitors for targeted correction and modulation of genetic dysfunctions in MPPH patients. Collaborations between academic institutes and biotech firms are also expanding the development pipeline for rare neurogenetic syndromes. As genetic testing becomes more accessible and personalized medicine advances, the potential for curative interventions in this segment continues to accelerate.
- By Route of Administration
On the basis of route of administration, the market is segmented into parenteral and others. The Parenteral segment dominated the market in 2024 with the largest share, owing to its use in delivering anti-seizure, neuroprotective, and supportive medications to MPPH patients, particularly in severe cases requiring immediate intervention. Parenteral routes ensure rapid absorption and effectiveness, especially in hospital-based management of acute neurological symptoms. The growing number of inpatient treatments and emergency care instances involving intravenous therapies supports this segment’s strength. In addition, hospital protocols favor parenteral delivery for controlled dosing in pediatric and critical care patients, ensuring treatment precision and safety.
The Others segment, which includes oral and topical routes, is expected to register the fastest growth during the forecast period due to growing adoption of maintenance and long-term supportive therapies. As patients stabilize, oral medications and nutritional supplements become integral for sustained neurological management. For instance, advancements in pediatric formulations have improved ease of administration for home-based care. This shift toward outpatient and self-managed treatments is propelling adoption of alternative routes for convenience, compliance, and quality-of-life improvement.
- By End-Users
On the basis of end-users, the market is segmented into hospitals, specialty clinics, and others. The Hospitals segment dominated the market in 2024, accounting for the largest revenue share due to the complex, multidisciplinary nature of MPPH Syndrome management. Hospitals provide comprehensive care through integrated departments of neurology, pediatrics, and genetics, offering advanced imaging, testing, and surgical facilities. The concentration of expertise and access to genetic counseling services further enhance diagnostic and treatment accuracy. Increased hospitalization rates for severe neurodevelopmental complications and the availability of specialized care units drive the dominance of this segment. The growing presence of rare-disease research centers within hospitals is also strengthening their role in patient care and clinical trials.
The Specialty Clinics segment is expected to grow at the fastest rate from 2025 to 2032, driven by the increasing establishment of dedicated neurogenetic and pediatric specialty centers worldwide. These clinics offer personalized treatment plans and continuous monitoring tailored to rare disease patients. For instance, genetic therapy and neurodevelopmental rehabilitation clinics are becoming central to the continuum of care in developed regions. The rise of outpatient-based genetic counseling and telehealth consultations is also expanding accessibility to specialized services, fostering growth of this segment in both developed and emerging economies.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and others. The Hospital Pharmacy segment dominated the market in 2024, owing to the high dependence of MPPH patients on hospital-based drug dispensing and controlled medication management. Hospital pharmacies are central in supplying anti-epileptic, neuroprotective, and supportive therapies that require prescription validation and monitoring by neurologists and geneticists. The concentration of rare disease treatments within tertiary care centers supports this channel’s market leadership. Hospital pharmacists also play a crucial role in maintaining drug safety and adherence protocols for pediatric and critical care patients.
The Retail Pharmacy segment is anticipated to witness the fastest growth from 2025 to 2032, fueled by the increasing availability of maintenance drugs, supplements, and follow-up therapies for home-based management of MPPH symptoms. For instance, retail chains are expanding their offerings of orphan and specialty drugs through improved supply chain networks and online ordering systems. Growing awareness among caregivers and increased accessibility to rare-disease medications through retail channels are strengthening this segment. The rise of e-pharmacy platforms and reimbursement support for long-term care medications further contribute to its accelerated growth trajectory.
Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market Regional Analysis
- North America dominated the MPPH Syndrome market with the largest revenue share of 41.8% in 2024, supported by strong rare-disease research networks, availability of advanced diagnostic infrastructure, and the presence of major academic and healthcare institutions focusing on early diagnosis and treatment innovation
- Patients in the region benefit from early access to next-generation sequencing, genetic counseling, and specialized neurological care offered by leading hospitals and research institutions
- This dominance is further supported by favorable government policies for orphan drug development, high healthcare expenditure, and active collaboration between academic centers and biotech companies, positioning North America as the primary hub for diagnosis, research, and treatment innovation in MPPH
U.S. Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market Insight
The U.S. MPPH Syndrome market captured the largest revenue share of 79% in 2024 within North America, fueled by robust investments in genomic research, advanced neuroimaging facilities, and strong rare-disease awareness programs. The nation’s leading research institutions, such as the NIH and major pediatric hospitals, are actively conducting studies on PI3K-AKT-MTOR pathway mutations. Increasing access to next-generation sequencing, coupled with favorable orphan drug policies, is accelerating early diagnosis and precision treatment development. Moreover, rising collaboration between biotech firms and clinical research networks is further driving the U.S. market’s leadership in the diagnosis and management of MPPH Syndrome.
Europe Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus (MPPH) Syndrome Market Insight
The Europe MPPH Syndrome market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by the growing focus on rare neurological disorders and cross-border research initiatives under EU health programs. The region’s advanced healthcare infrastructure, combined with strong regulatory support for orphan drug designation, encourages active participation from research institutions and pharmaceutical companies. Increasing adoption of genetic testing and clinical trials across countries such as Germany, France, and the U.K. is enhancing patient identification. Europe’s strategic emphasis on data sharing and digital health platforms continues to foster regional market expansion.
U.K. Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus (MPPH) Syndrome Market Insight
The U.K. MPPH Syndrome market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by its well-established genomic infrastructure and national health initiatives focused on rare disease management. The country’s Genomics England project plays a vital role in advancing early genetic screening and personalized medicine. For instance, increased clinical collaborations between NHS facilities and academic institutions are enhancing diagnostic capabilities for complex neurological syndromes. In addition, government funding for rare-disease research and expanding access to specialized clinics continue to stimulate the market’s growth trajectory.
Germany Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus (MPPH) Syndrome Market Insight
The Germany MPPH Syndrome market is expected to expand at a considerable CAGR during the forecast period, supported by strong medical research networks, advanced neurodiagnostic technologies, and active participation in European rare-disease consortia. German hospitals are investing in precision neurology and genome sequencing programs to better understand cortical malformation disorders. For instance, academic partnerships with biotech firms are fostering research into molecular mechanisms underlying brain overgrowth syndromes. The country’s commitment to innovation and patient-centered care is driving the adoption of advanced diagnostic tools and gene-based research initiatives.
Asia-Pacific Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus (MPPH) Syndrome Market Insight
The Asia-Pacific MPPH Syndrome market is poised to grow at the fastest CAGR of 23.5% during the forecast period of 2025 to 2032, driven by growing awareness of rare genetic disorders and expanding access to diagnostic technologies in countries such as Japan, China, and India. Regional healthcare modernization and the rapid development of genetic testing centers are accelerating early detection. In addition, government-led rare disease registries and collaborative clinical studies are improving patient management. As APAC emerges as a center for affordable genomic testing and precision medicine, the market is expected to expand steadily across both developed and emerging economies.
Japan Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus (MPPH) Syndrome Market Insight
The Japan MPPH Syndrome market is gaining momentum due to the country’s advanced biotechnology ecosystem, strong academic research base, and commitment to rare-disease genomics. Increasing integration of AI in diagnostic imaging and genetic interpretation is enhancing detection accuracy for MPPH and related disorders. For instance, national programs promoting genetic data digitization are supporting long-term disease monitoring. Japan’s aging population and emphasis on precision healthcare are also stimulating demand for advanced diagnostic and therapeutic solutions across hospitals and specialty clinics.
India Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus (MPPH) Syndrome Market Insight
The India MPPH Syndrome market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to rapid healthcare infrastructure growth, government-led genomics initiatives, and expanding access to rare-disease diagnostics. Increasing collaborations between research institutes and global genomic networks are enhancing early detection rates. For instance, national health programs promoting rare disease registries and gene mapping have improved patient identification and referral systems. The rising availability of affordable genetic testing and the growing presence of local biotech companies are key factors propelling market development in India.
Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market Share
The Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- Illumina, Inc. (U.S.)
- Invitae Corporation (U.S.)
- Natera, Inc. (U.S.)
- GeneDx, LLC (U.S.)
- Fulgent Genetics, Inc. (U.S.)
- Baylor Genetics (U.S.)
- PreventionGenetics (U.S.)
- Eurofins Genomics (Germany)
- Myriad Genetics, Inc. (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Labcorp (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- AstraZeneca (U.K.)
- Sanofi. (France)
- BridgeBio Pharma, Inc. (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
What are the Recent Developments in Global Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome Market?
- In March 2025, a broad study expanded the phenotypic and genetic spectrum of AKT3-related neurodevelopmental disorders, showing more diverse clinical presentations and clarifying genotype–phenotype correlations (including AKT3 changes relevant to MPPH-such as presentations). This work improves genetic diagnosis, counseling, and the interpretation of AKT3 variants in patients with brain overgrowth syndromes
- In May 2024, researchers reported a de novo truncating mutation in CCND2 (Cyclin D2) associated with megalencephaly and polymicrogyria, adding evidence that CCND2 variants can underlie MPPH-spectrum phenotypes and expanding the list of causal genes beyond AKT3/PIK3R2/PIK3CA. That finding helped clinicians broaden gene panels and reinforced the role of cell-cycle regulators in brain overgrowth disorders
- In November 2023, a case report described prenatal ultrasound detection of fetal megalencephaly with subsequent whole-exome sequencing confirmation of an AKT3 mutation, demonstrating that MPPH can be suspected prenatally and that genomic testing (WES) can confirm diagnosis before birth — important for prenatal counselling and perinatal management planning
- In October 2023, a genetics/oncogenetics report described a somatic (post-zygotic) AKT3 p.T81dup variant that produced a mild cerebral overgrowth phenotype and capillary malformation, expanding the known AKT3 mutation spectrum and underscoring the importance of searching for mosaic/somatic variants in brain overgrowth syndromes
- In June 2021, a detailed case report and literature review summarized clinical features, neuroimaging, and the genetic causes (AKT3, PIK3R2, PIK3CA, CCND2) of MPPH, consolidating evidence that PI3K-AKT pathway dysregulation drives these syndromes and encouraging standardized reporting of genotype and detailed imaging to improve phenotype–genotype mapping. This paper has been frequently cited in later genetic and clinical updates
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.