Global Mepolizumab Market
Market Size in USD Billion
CAGR :
% 
 USD
2.31 Billion
USD
3.81 Billion
2024
2032
USD
2.31 Billion
USD
3.81 Billion
2024
2032
| 2025 –2032 | |
| USD 2.31 Billion | |
| USD 3.81 Billion | |
|
|
|
|
Mepolizumab Market Size
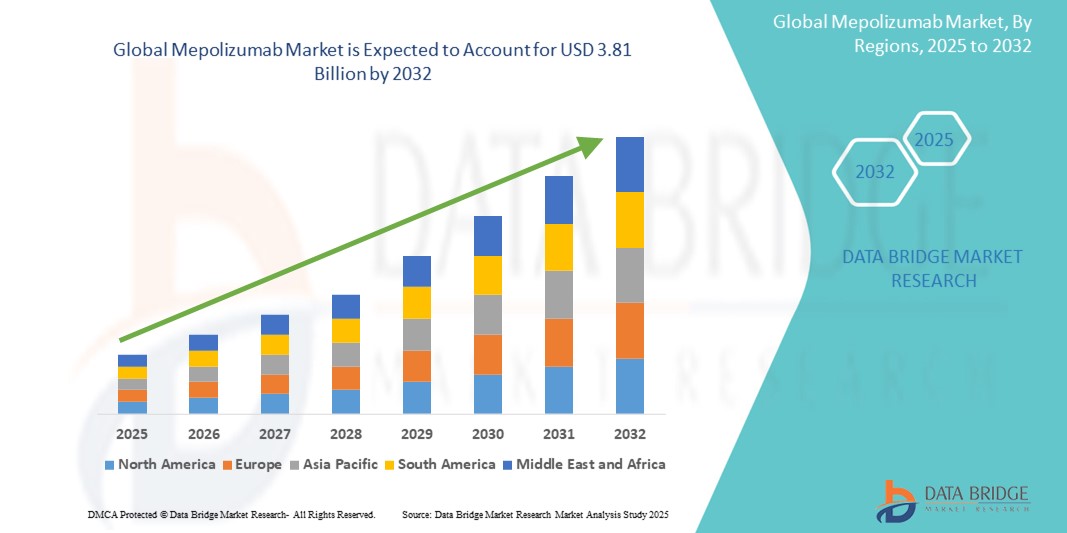
- The global mepolizumab market size was valued at USD 2.31 billion in 2024 and is expected to reach USD 3.81 billion by 2032, at a CAGR of 6.42% during the forecast period
- This growth is driven by factors such as rising prevalence of severe eosinophilic asthma and other eosinophil-related disorders
Mepolizumab Market Analysis
- Mepolizumab is a humanized monoclonal antibody targeting interleukin-5 (IL-5), primarily used in the treatment of eosinophilic-driven conditions such as severe eosinophilic asthma, EGPA (eosinophilic granulomatosis with polyangiitis), hypereosinophilic syndrome (HES), and chronic rhinosinusitis with nasal polyps (CRSwNP). It works by reducing eosinophil levels, which play a key role in inflammation and disease severity
- The global mepolizumab market is experiencing steady growth due to rising awareness and diagnosis of eosinophilic disorders, expanded regulatory approvals for multiple indications, and increased adoption of biologics in asthma and autoimmune disease management
- North America is expected to dominate the mepolizumab market with a market share of approximately 48.50%, driven by advanced healthcare infrastructure, high adoption rates of biologic therapies, and the strong presence of key pharmaceutical players
- Asia-Pacific is expected to be the fastest growing region in the mepolizumab market during the forecast period, driven by rapid expansion in healthcare infrastructure, increasing awareness about eosinophilic disorders, and growing adoption of biologic treatments
- Severe asthma segment is expected to dominate the market with a market share of 62.65% due to its high prevalence and established clinical use of biologic therapies. As a primary indication for mepolizumab, severe eosinophilic asthma accounts for the majority of prescriptions, supported by strong clinical evidence and guideline recommendations
Report Scope and Mepolizumab Market Segmentation
|
Attributes |
Mepolizumab Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Mepolizumab Market Trends
“Expanding Use of Biologics for Personalized Treatment of Eosinophilic-Driven Diseases”
- One prominent trend in the evolution of the global mepolizumab market is the expanding use of biologics for personalized treatment of eosinophilic-driven diseases
- This shift reflects a growing preference for targeted therapies that offer improved efficacy and safety profiles compared to conventional treatments, especially in managing severe asthma and rare eosinophilic disorders
- For instance, mepolizumab’s ability to selectively inhibit interleukin-5 (IL-5) has led to its adoption in treating conditions such as hypereosinophilic syndrome (HES) and EGPA, with ongoing clinical trials exploring its utility in other IL-5–mediated conditions
- This targeted treatment approach is transforming the management of eosinophilic diseases, enabling more personalized care, reducing corticosteroid dependence, and fueling global demand for monoclonal antibody therapies such as mepolizumab
Mepolizumab Market Dynamics
Driver
“Rising Prevalence of Severe Eosinophilic Asthma and Other Eosinophil-Related Disorders”
- The rising prevalence of severe eosinophilic asthma and other eosinophil-related disorders is significantly driving the demand for biologic therapies such as mepolizumab
- As global awareness of eosinophilic phenotypes increases, especially among patients unresponsive to standard asthma treatments, there is growing recognition of the need for targeted biologics that improve disease control and reduce exacerbations
- This demand is further supported by the increasing availability of diagnostic tools that identify eligible patients based on blood eosinophil counts and clinical history
For instance,
- According to the Global Asthma Report 2022, over 339 million people worldwide suffer from asthma, with a significant subset exhibiting eosinophilic inflammation, highlighting a substantial market opportunity for targeted IL-5 inhibitors such as mepolizumab
- As a result, the growing prevalence of eosinophilic conditions and advancements in biomarker-driven diagnostics are fueling the uptake of biologics, accelerating the global growth of the mepolizumab market
Opportunity
“Expansion Of Mepolizumab into New Indications”
- The expansion of mepolizumab into new indications beyond asthma presents a significant growth opportunity in the biologics market
- With its proven efficacy in targeting interleukin-5 (IL-5), mepolizumab is being explored for a broader range of eosinophil-associated conditions, offering the potential to address unmet medical needs in rare and underserved diseases
- In addition, continued clinical trials and regulatory approvals in emerging indications could enable mepolizumab to capture new patient populations and drive market expansion
For instance,
- In September 2020, the U.S. FDA approved mepolizumab for the treatment of hypereosinophilic syndrome (HES) in patients aged 12 years and older, marking the first biologic approved for HES in over a decade. This milestone demonstrates the drug's versatility and opens the door for future applications in other eosinophil-driven diseases, such as nasal polyposis and atopic dermatitis
- The expansion into new therapeutic areas not only strengthens mepolizumab's market position but also enhances access to targeted therapy for patients with rare and chronic eosinophilic conditions, fueling long-term growth opportunities for biopharmaceutical companies
Restraint/Challenge
“Limited awareness and delayed diagnosis of Eosinophilic Conditions”
- Limited awareness and delayed diagnosis of eosinophilic conditions significantly hinder the optimal use of mepolizumab in many regions
- Despite its proven efficacy, many patients with severe eosinophilic asthma, EGPA, or HES remain undiagnosed or misdiagnosed due to a lack of specialized testing, trained professionals, and public health education
- This gap in early and accurate diagnosis prevents timely initiation of targeted biologic therapy, reducing patient outcomes and curbing potential market growth
For instance,
- A study published in the Journal of Asthma and Allergy in 2023 highlighted that nearly 40% of patients with eosinophilic asthma remain undiagnosed or improperly classified, even in developed healthcare systems, delaying access to appropriate treatment options such as mepolizumab
- As a result, underdiagnosis and clinical unawareness remain key obstacles, limiting the reach of mepolizumab and slowing its adoption across global markets
Mepolizumab Market Scope
The market is segmented on the basis of indication, age group, distribution channel and end user.
|
Segmentation |
Sub-Segmentation |
|
By Indication |
|
|
By Age Group |
|
|
By Distribution Channel |
|
|
By End User |
|
In 2025, the severe asthma is projected to dominate the market with a largest share in indication segment
The severe asthma segment is expected to dominate the global mepolizumab market with the largest share of approximately 62.65% in 2025 due to its high prevalence and established clinical use of biologic therapies. As a primary indication for mepolizumab, severe eosinophilic asthma accounts for the majority of prescriptions, supported by strong clinical evidence and guideline recommendations. The increasing global burden of asthma, coupled with growing adoption of precision medicine and targeted biologics, continues to drive demand, reinforcing this segment's market leadership.
The adults (18 years and older) is expected to account for the largest share during the forecast period in age group market
In 2025, the adults (18 years and older) segment is expected to dominate the global mepolizumab market with the largest market share of 58.4% due to the higher prevalence of severe eosinophilic asthma and other eosinophil-driven conditions in this age group. Adults represent the largest patient population for biologic treatments such as mepolizumab, particularly for managing chronic conditions such as severe asthma, EGPA, and chronic rhinosinusitis. The increasing adoption of biologics for personalized treatment, along with the growing recognition of the effectiveness of mepolizumab in adult patients, continues to drive market growth, solidifying this segment's dominance.
Mepolizumab Market Regional Analysis
“North America Holds the Largest Share in the Mepolizumab Market”
- North America dominates the global mepolizumab market with a market share of approximately 48.50%, driven by advanced healthcare infrastructure, high adoption rates of biologic therapies, and the strong presence of key pharmaceutical players
- U.S. holds a major market share, due to the high prevalence of severe eosinophilic asthma, widespread use of biologic treatments, and well-established reimbursement systems
- The presence of comprehensive health insurance plans and government healthcare programs such as Medicare and Medicaid ensure broader access to mepolizumab for eligible patients, particularly in asthma and other eosinophilic disorders
- Furthermore, ongoing research into the efficacy of mepolizumab for additional indications such as eosinophilic granulomatosis with polyangiitis (EGPA) and chronic rhinosinusitis with nasal polyps (CRSwNP) continues to drive market growth across North America
- This robust healthcare environment and growing patient population seeking advanced treatments further strengthen the market’s dominance in North America
“Asia-Pacific is Projected to Register the Highest CAGR in the Mepolizumab Market”
- Asia-Pacific is expected to witness the highest growth rate in the global mepolizumab market, driven by rapid expansion in healthcare infrastructure, increasing awareness about eosinophilic disorders, and growing adoption of biologic treatments
- Countries such as China, India, and Japan are emerging as key markets due to rising healthcare access, expanding middle-class populations, and increasing demand for advanced treatment options for asthma and other eosinophil-related diseases
- Japan remains a key market with its advanced healthcare system, widespread adoption of biologic therapies, and high demand for precision medicine. The country has a strong regulatory framework that facilitates the approval and reimbursement of biologic treatments, positioning it as a leader in the uptake of mepolizumab and similar therapies
- India is expected to register the highest CAGR with 9.80% market share in the mepolizumab market, driven by an expanding healthcare infrastructure, a rising burden of respiratory diseases such as severe asthma, and increasing access to advanced biologic therapies. In addition, rising awareness about eosinophilic conditions and improving healthcare affordability further contribute to the market's growth in India
Mepolizumab Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- AstraZeneca (U.K.)
- Boehringer Ingelheim International GmbH (Germany)
- GSK plc. (U.K.)
- Novartis AG (Switzerland)
- Regeneron Pharmaceuticals Inc. (U.S.)
- Sanofi (France)
- Teva Pharmaceutical Industries Ltd. (Israel)
Latest Developments in Global Mepolizumab Market
- In April 2025, GSK plc reported positive results for Nucala (mepolizumab) in treating chronic obstructive pulmonary disease (COPD), with the complete findings from the MATINEE phase III trial published in the New England Journal of Medicine. The trial assessed mepolizumab, a monoclonal antibody targeting interleukin-5 (IL-5), in a diverse group of COPD patients. Across the entire study population, mepolizumab demonstrated a clinically meaningful and statistically significant 21% reduction in the annualized rate of moderate/severe exacerbations compared to placebo, successfully meeting the primary endpoint of the MATINEE trial
- In December 2024, GSK plc announced positive results for Nucala (mepolizumab) in the treatment of chronic obstructive pulmonary disease (COPD), with the full findings from the MATINEE phase III trial published in the New England Journal of Medicine. The trial assessed mepolizumab, a monoclonal antibody targeting interleukin-5 (IL-5), in a broad range of COPD patients, including those with the most severe and hard-to-treat forms, as classified by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines. Nucala is currently approved for use in the U.S. for four IL-5 mediated conditions
- In January 2024, GSK plc announced that the China National Medical Products Administration (NMPA) had approved Nucala (mepolizumab) as an add-on maintenance treatment for severe eosinophilic asthma in adults and adolescents aged 12 years and older. This marks Nucala as the first anti-Interleukin-5 (IL-5) targeting treatment to receive approval in China for use in adult and adolescent patients with this condition
- In July 2021, GlaxoSmithKline plc (GSK) announced that the U.S. Food and Drug Administration (FDA) had approved Nucala (mepolizumab), a monoclonal antibody targeting interleukin-5 (IL-5), as a treatment for chronic rhinosinusitis with nasal polyps (CRSwNP). This approval provides a new indication for mepolizumab as an add-on maintenance treatment for adult patients aged 18 and older with CRSwNP who have not responded adequately to nasal corticosteroids
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.