Global Merkel Cell Carcinoma Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
3.24 Billion
USD
4.39 Billion
2021
2029
USD
3.24 Billion
USD
4.39 Billion
2021
2029
| 2022 –2029 | |
| USD 3.24 Billion | |
| USD 4.39 Billion | |
|
|
|
|
Merkel Cell Carcinoma Treatment Market Analysis and Size
The merkel cell carcinoma treatment market is expected to witness significant growth during the forecast period. After melanoma, Merkel cell carcinoma is the 2nd most widespread cause of skin cancer death. This cancer is caused by a weakened immune system and extreme sun exposure. Strong immunotherapy uptake and favourable reimbursement are encouraging indicators for Merkel cell carcinoma treatment growth. The endlessly improving healthcare infrastructure and the rise in people's disposable incomes in Asia-Pacific region are expected to increase the market in the forecast period. COVID-19 also had a major impact on the market growth.
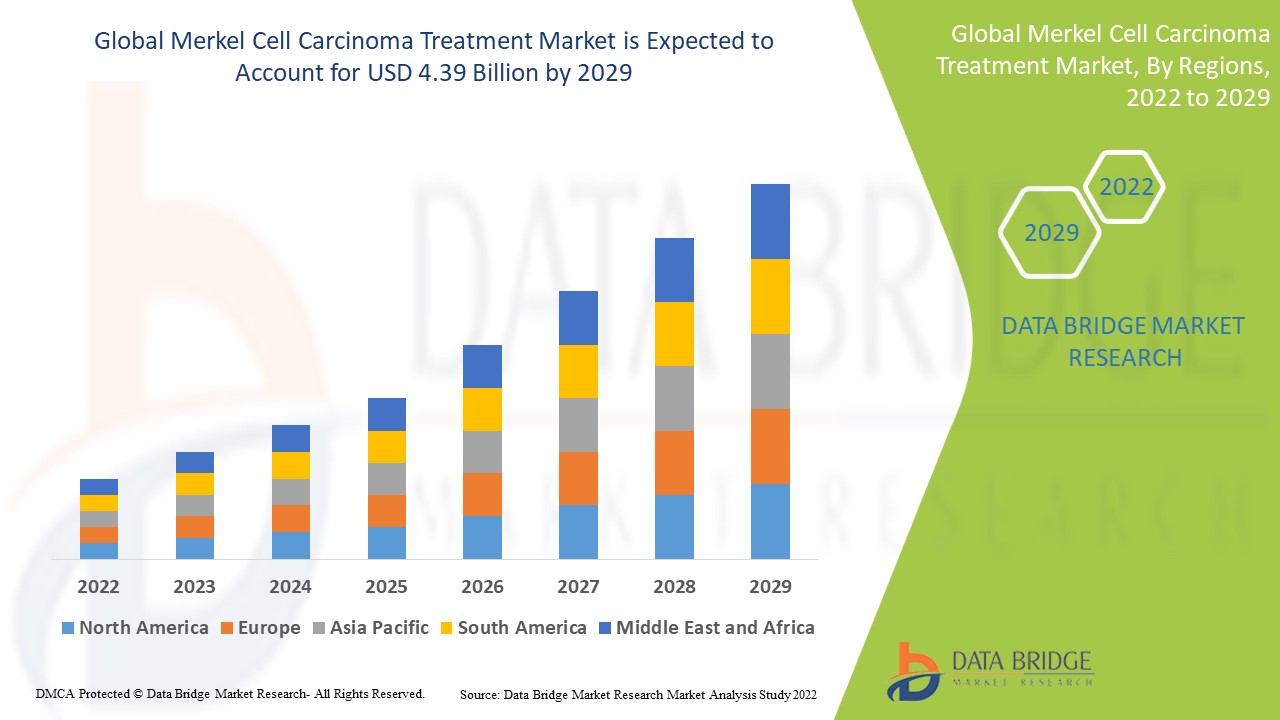
Data Bridge Market Research analyses a growth rate in the merkel cell carcinoma treatment market in the forecast period 2022-2029. The expected CAGR of merkel cell carcinoma treatment market is tend to be around 3.87% in the mentioned forecast period. The market was valued at USD 3.24 billion in 2021, and it would grow upto USD 4.39 billion by 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Merkel Cell Carcinoma Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Treatment Type (Immunotherapy, Chemotherapy, Others), Route of Administration (Oral, Parenteral), End-Users (Hospitals, Homecare, Speciality Centres, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Johnson & Johnson Private Limited (U.S.), Cipla Inc. (U.S.), Abbott (U.S.), AbbVie Inc. (U.S.), Merck KGaA (Germany), Sun Pharmaceutical Industries Ltd. (India), Aurobindo Pharma (India), Lupin (India), Hikma Pharmaceuticals PLC (U.K.), Amneal Pharmaceuticals LLC. (U.S.), Pfizer Inc (U.S.), Mylan N.V. (U.S.), Novartis AG (Switzerland), Bristol-Myers Squibb Company (U.S.), GSK plc. (U.K.), Bayer AG (Germany), ImmunityBio, Inc (U.S.), Oncovir, Inc (U.S.) |
|
Market Opportunities |
|
Market Definition
Merkel cell carcinoma treatment is also known as neuroendocrine carcinoma of the skin a rare and aggressive type of skin cancer that originates from the top layers of the skin. It is caused either by continued exposure to sun, weak immunity or maybe others idiopathic reasons. Weak immune systems and sun exposure encourage the effects of merkel cell carcinoma. MCC generally appears as a single painless lump when exposed to the sun. It is of great importance to the healthcare sector and thus is expected to rise high in the forecast period.
Global Merkel Cell Carcinoma Treatment Market Dynamics
Drivers
- Increased Research Activities
Increasing research and development activities for the treatment of merkel cell carcinoma is projected to boost the market. In May 2017, researchers of Fred Hutchison University and the University of Washington in Seattle stated the results of a small study combining Avelumab with two other treatments. The combination therapy helps to improve the T cells generation and attacks the MCC cells. The National Cancer Institute has also began a clinical trial that involves the investigational study of the drug ipilimumab Merkel cell carcinoma. This boosts the market growth.
- Rising Demand for Oral Drugs
Oral drugs is expected to boost the market growth. The segment is expected to accelerate the global merkel cell carcinoma treatment market as most products are available in capsule and tablet form and it is a very feasible route of administration.
Opportunities
- Increased and Innovative Diagnostic Tools
Numerous tests and procedures can be performed to examine the skin to detect and diagnose carcinoma cells; however, certain factors of prognosis and treatment options decline the chances of recovery. Several new developments in skin biopsy approaches enable the diagnosis of diseases or infection at the cellular and molecular level, making way for early diagnosis and treatment of diseases.
- High-end Conduction of Clinical Trials
The merkel cell carcinoma pipeline is projected to boost the market. For instance, in December 2018, Merck Sharp & Dohme Corp., a Merck & Co., Inc. subsidiary, undertook a phase 3 open-label, single-arm study to assess the efficacy of pembrolizumab as first-line therapy in the candidates suffering from advanced merkel cell carcinoma. Also, in December 2018, Incyte Corporation undertook a phase 2 study of INCMGA00012 in candidates with metastatic Merkel cell carcinoma. This creates more opportunity in the market.
Restraints/Challenges
- Lack of Skilled Professionals
Lack of trained professionals who are not aware of the appropriate treatment methods for this disease could restrain the growth of the global merkel cell carcinoma treatment market over a forecast period.
- Side-effects of Varied Treatments
There are several side-effects associated with merkel cell carcinoma treatment that hamper the growth of the market. Side-effects of external radiation therapy include skin changes, diarrhoea, painful sores in the throat and mouth, nausea, fatigue, and dry mouth or thick saliva. The side-effects of the radiation thaerapy are temporary; however, some rare serious side-effects could become permanent. In a few cases, radiation to the chest could even cause lung damage, which may lead to breathing issues and shortness of breath.
This global merkel cell carcinoma treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global merkel cell carcinoma treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Merkel Cell Carcinoma Treatment Market
The present Covid-19 pandemic has had a significant effect on all enterprises. The lack of beds to accommodate the needs of the growing number of patients was causing a medical aid shortage in the healthcare sector. The majority of enterprises are at a standstill, and those operating close to people's homes are barely able to handle revenue, which is causing the global economy to fluctuate. A major effect of the COVID-19 pandemic has been a delay in the treatment of intestine cancer, which can have harmful consequences for the overall and disease-free survival of patients. With the decline in COVID-19 cases, the number of referrals for intestine cancer treatment has reduced significantly.
Global Merkel Cell Carcinoma Treatment Market Scope
The global merkel cell carcinoma treatment market is segmented on the basis of treatment type, route of administration, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Treatment Type
- Immunotherapy
- Chemotherapy
- Others
Route of Administration
- Oral
- Parenteral
End-Users
- Hospitals
- Homecare
- Speciality Centres
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Merkel Cell Carcinoma Treatment Market Regional Analysis/Insights
The global merkel cell carcinoma treatment market is analysed and market size insights and trends are provided by treatment type, route of administration, distribution channel and end-user as referenced above.
The major countries covered in the global merkel cell carcinoma treatment market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to have the highest market growth due to the rise cases of skin cancer and favourable government policies, and advanced healthcare facilities.
Asia-Pacific dominates the market due to the increase government initiatives and rapidly aging population.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Merkel Cell Carcinoma Treatment Market Share Analysis
The global merkel cell carcinoma treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global merkel cell carcinoma treatment market.
Key players operating in the global merkel cell carcinoma treatment market include:
- Johnson & Johnson Private Limited (U.S.)
- Cipla Inc. (U.S.)
- Abbott (U.S.)
- AbbVie Inc. (U.S.)
- Merck KGaA (Germany)
- Sun Pharmaceutical Industries Ltd. (India)
- Aurobindo Pharma (India)
- Lupin (India)
- Hikma Pharmaceuticals PLC (U.K.)
- Amneal Pharmaceuticals LLC. (U.S.)
- Pfizer Inc (U.S.)
- Mylan N.V. (U.S.)
- Novartis AG (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- GSK plc. (U.K.)
- Bayer AG (Germany)
- ImmunityBio, Inc (U.S.)
- Oncovir, Inc (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTLE
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 EPIO NUMBER
6.1.4 PATENT STRENGTH AND QUALITY
6.1.5 PATENT CLAIMS
6.1.6 PATENT CITATIONS
6.1.7 PATENT LITIGATION AND LICENSING
6.1.8 FILE OF PATENT
6.1.9 PATENT RECEIVED CONTRIES
6.1.10 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOT
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 PHASE III CANDIDATES
9.2 PHASE II CANDIDATES
9.3 PHASE I CANDIDATES
9.4 OTHERS (PRE-CLINICAL AND RESEARCH)
10 MARKET ACCESS
10.1 10-YEAR MARKET FORECAST
10.2 CLINICAL TRIAL RECENT UPDATES
10.3 ANNUAL NEW FDA APPROVED DRUGS
10.4 DRUGS MANUFACTURER AND DEALS
10.5 MAJOR DRUG UPTAKE
10.6 CURRENT TREATMENT PRACTICES
10.7 IMPACT OF UPCOMING THERAPY
11 R & D ANALYSIS
11.1 COMPARATIVE ANALYSIS
11.2 DRUG DEVELOPMENTAL LANDSCAPE
11.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
11.4 THERAPEUTIC ASSESSMENT
11.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
11.6 DRUGS AND THERAPIES IN CLINICAL TRIALS
11.7 EMERGING TREATMENT APPROACHES
12 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, BY TREATMENT TYPE
12.1 OVERVIEW
12.2 IMMUNOTHERAPY
12.2.1 IMMUNE CHECKPOINT INHIBITORS
12.2.1.1. PD-1 AND PD-L1 INHIBITOR THERAPY
12.2.1.2. CTLA-4 INHIBITOR THERAPY
12.2.1.3. OTHERS
12.2.2 CYTOKINES
12.2.2.1. INTERFERONS
12.2.2.1.1. PEGYLATED INTERFERON-ALPHA (PEG-IFN-Α)
12.2.2.1.2. INTERFERON-ALPHA (IFN-Α)
12.2.2.2. INTERLEUKINS (ILS)
12.2.2.3. OTHERS
12.3 CHEMOTHERAPY
12.3.1 PLATINUM-BASED CHEMOTHERAPY
12.3.1.1. CISPLATIN
12.3.1.2. CARBOPLATIN
12.3.2 TOPOISOMERASE INHIBITORS
12.3.2.1. TOPOISOMERASE I INHIBITORS
12.3.2.1.1. IRINOTECAN
12.3.2.1.2. TOPOTECAN
12.3.2.1.3. OTHERS
12.3.2.2. TOPOISOMERASE II INHIBITORS
12.3.2.2.1. ETOPOSIDE
12.3.2.2.2. DOXORUBICIN
12.3.3 ANTIMETABOLITES
12.3.3.1. FLUOROURACIL (5-FU)
12.3.3.2. METHOTREXATE
12.3.4 OTHERS
12.4 RADIATION THERAPY
12.5 SURGERY
12.5.1 WIDE LOCAL EXCISION
12.5.2 LYMPH NODE DISSECTION
12.5.2.1. INGUINAL LYMPH NODE DISSECTION
12.5.2.2. REGIONAL LYMPH NODE DISSECTION
12.5.3 MOHS MICROGRAPHIC SURGERY
12.5.4 RECONSTRUCTIVE SURGERY
12.5.5 OTHERS
13 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, BY DRUGS TYPE
13.1 OVERVIEW
13.2 BRANDED
13.2.1 AVELUMAB
13.2.2 PEMBROLIZUMAB
13.2.3 RETIFANLIMAB-DLWR
13.2.4 ZYNYZ (RETIFANLIMAB-DLWR)
13.2.5 OTHERS
13.3 GENERICS
14 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, BY STAGES
14.1 OVERVIEW
14.2 STAGE I
14.3 STAGE II
14.4 STAGE III
14.5 STAGE IV
15 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 ORAL
15.2.1 SOLID
15.2.1.1. PILL
15.2.1.2. CAPSULE
15.2.1.3. TABLETS
15.2.1.4. OTHERS
15.2.2 LIQUID
15.2.3 OTHERS
15.3 PARENTERAL
15.3.1 INTRAVENOUS
15.3.2 SUBCUTANEOUS
15.3.3 INTRAMUSCULAR
16 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, BY GENDER
16.1 OVERVIEW
16.2 MALE
16.3 FEMALE
17 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITAL
17.3 CLINICS
17.4 HOME HEALTHCARE
17.5 DIAGNOSTIC CENTERS
17.6 OTHERS
18 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 DIRECT TENDER
18.3 HOSPITALS PHARMACY
18.4 RETAIL PHARMACY
18.5 ONLINE PHARMACY
18.6 OTHERS
19 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, COMPANY LANDSCAPE
19.1 COMPANY SHARE ANALYSIS: GLOBAL
19.2 MERGERS & ACQUISITIONS
19.3 NEW PRODUCT DEVELOPMENT & APPROVALS
19.4 EXPANSIONS
19.5 REGULATORY CHANGES
19.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
20 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, SWOT AND DBMR ANALYSIS
21 GLOBAL MERKEL CELL CARCINOMA TREATMENT MARKET, COMPANY PROFILE
21.1 MARKETED DRUGS COMPANIES
21.1.1 PFIZER INC./MERCK KGAA
21.1.1.1. COMPANY OVERVIEW
21.1.1.2. REVENUE ANALYSIS
21.1.1.3. GEOGRAPHIC PRESENCE
21.1.1.4. PRODUCT PORTFOLIO
21.1.1.5. RECENT DEVELOPEMENTS
21.1.2 BRISTOL-MYERS SQUIBB BELGIUM N/V
21.1.2.1. COMPANY OVERVIEW
21.1.2.2. REVENUE ANALYSIS
21.1.2.3. GEOGRAPHIC PRESENCE
21.1.2.4. PRODUCT PORTFOLIO
21.1.2.5. RECENT DEVELOPEMENTS
21.1.3 INCYTE
21.1.3.1. COMPANY OVERVIEW
21.1.3.2. REVENUE ANALYSIS
21.1.3.3. GEOGRAPHIC PRESENCE
21.1.3.4. PRODUCT PORTFOLIO
21.1.3.5. RECENT DEVELOPEMENTS
21.2 PIPELINE DRUGS COMPANIES
21.2.1 KARTOS THERAPEUTICS, INC
21.2.1.1. COMPANY OVERVIEW
21.2.1.2. REVENUE ANALYSIS
21.2.1.3. GEOGRAPHIC PRESENCE
21.2.1.4. PRODUCT PORTFOLIO
21.2.1.5. RECENT DEVELOPEMENTS
21.2.2 4SC AG
21.2.2.1. COMPANY OVERVIEW
21.2.2.2. REVENUE ANALYSIS
21.2.2.3. GEOGRAPHIC PRESENCE
21.2.2.4. PRODUCT PORTFOLIO
21.2.2.5. RECENT DEVELOPEMENTS
21.2.3 ONCOVIR, INC
21.2.3.1. COMPANY OVERVIEW
21.2.3.2. REVENUE ANALYSIS
21.2.3.3. GEOGRAPHIC PRESENCE
21.2.3.4. PRODUCT PORTFOLIO
21.2.3.5. RECENT DEVELOPEMENTS
21.2.4 SOTIO BIOTECH B.V.
21.2.4.1. COMPANY OVERVIEW
21.2.4.2. REVENUE ANALYSIS
21.2.4.3. GEOGRAPHIC PRESENCE
21.2.4.4. PRODUCT PORTFOLIO
21.2.4.5. RECENT DEVELOPEMENTS
21.2.5 IMMUNITYBIO, INC.
21.2.5.1. COMPANY OVERVIEW
21.2.5.2. REVENUE ANALYSIS
21.2.5.3. GEOGRAPHIC PRESENCE
21.2.5.4. PRODUCT PORTFOLIO
21.2.5.5. RECENT DEVELOPEMENTS
21.2.6 IMMUNOMIC THERAPEUTICS, INC
21.2.6.1. COMPANY OVERVIEW
21.2.6.2. REVENUE ANALYSIS
21.2.6.3. GEOGRAPHIC PRESENCE
21.2.6.4. PRODUCT PORTFOLIO
21.2.6.5. RECENT DEVELOPEMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
22 RELATED REPORTS
23 CONCLUSION
24 QUESTIONNAIRE
25 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.