Global Methylmalonic Acidemia Market
Market Size in USD Billion
CAGR :
% 
 USD
9.89 Billion
USD
14.94 Billion
2024
2032
USD
9.89 Billion
USD
14.94 Billion
2024
2032
| 2025 –2032 | |
| USD 9.89 Billion | |
| USD 14.94 Billion | |
|
|
|
|
Methylmalonic Acidemia Market Size
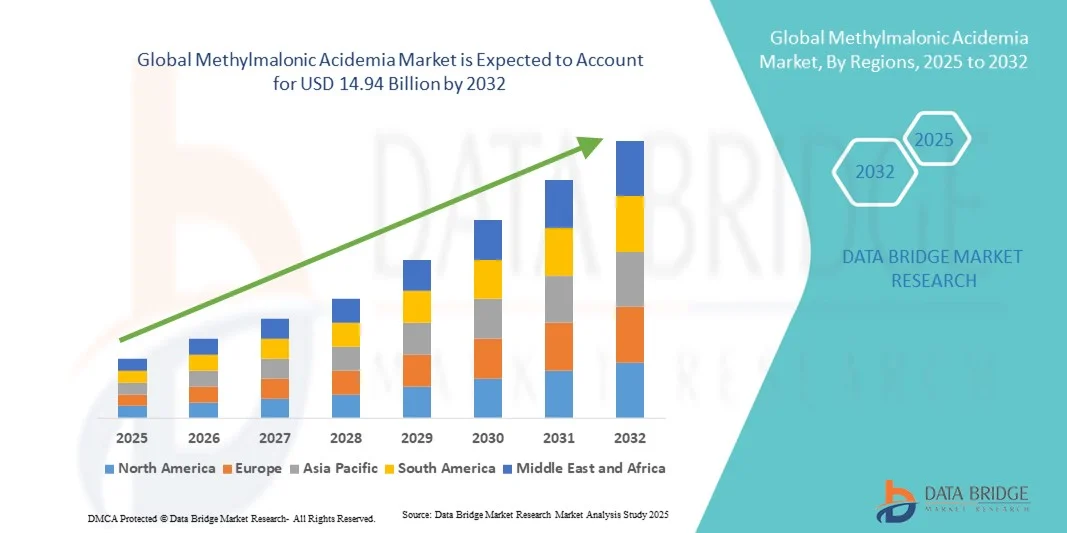
- The global methylmalonic acidemia market size was valued at USD 9.89 billion in 2024 and is expected to reach USD 14.94 billion by 2032, at a CAGR of 5.30% during the forecast period
- The market growth is largely fueled by the growing advancements in genetic research, molecular diagnostics, and enzyme replacement therapies, leading to improved detection and management of Methylmalonic Acidemia (MMA) across global healthcare systems
- Furthermore, rising awareness among healthcare providers, the introduction of newborn screening programs, and increasing investment in rare disease research are establishing Methylmalonic Acidemia as a key focus area in the metabolic disorder treatment landscape. These converging factors are accelerating the adoption of advanced diagnostic and therapeutic solutions, thereby significantly boosting the industry’s growth
Methylmalonic Acidemia Market Analysis
- Methylmalonic Acidemia (MMA), a rare inherited metabolic disorder caused by a deficiency of the enzyme methylmalonyl-CoA mutase, is gaining significant research and clinical attention due to advancements in genetic diagnostics, newborn screening, and enzyme replacement therapies
- The growing demand for early detection and treatment of MMA is primarily fueled by the increasing availability of molecular testing technologies, expanding healthcare access in emerging markets, and rising awareness among physicians and caregivers
- North America dominated the methylmalonic acidemia market with the largest revenue share of 41.5% in 2024, supported by advanced genetic research infrastructure, strong funding for rare disease studies, and the presence of key biotechnology and pharmaceutical companies focused on metabolic disorders. The U.S. leads the region, driven by early adoption of gene therapies, active clinical trials, and government initiatives for rare disease management
- Asia-Pacific is expected to be the fastest-growing region in the methylmalonic acidemia market during the forecast period, registering a high CAGR from 2025 to 2032. This growth is driven by improving healthcare systems, increasing genomic research capabilities, and growing government support for rare disease screening and treatment programs across countries such as China, Japan, and India
- The isolated methylmalonic acidurias segment held the largest market revenue share of 56.3% in 2024, driven by the comparatively higher incidence rate and established diagnostic and therapeutic frameworks supporting its management.
Report Scope and Methylmalonic Acidemia Market Segmentation
|
Attributes |
Methylmalonic Acidemia Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Methylmalonic Acidemia Market Trends
Increasing Focus on Early Diagnosis and Precision Therapies
- A significant and accelerating trend in the global methylmalonic acidemia market is the growing focus on early diagnosis through newborn screening programs and advancements in genetic testing. These efforts are enabling earlier detection of MMA, improving patient outcomes through prompt dietary management and therapeutic interventions
- For instance, in January 2024, researchers developed an improved tandem mass spectrometry (MS/MS) screening method for MMA, allowing faster and more accurate identification of metabolic abnormalities in infants. This innovation has enhanced early intervention success rates and reduced long-term neurological complications
- Advances in artificial intelligence (AI)-driven diagnostics are also improving clinical interpretation of complex metabolic data. AI-enabled platforms assist clinicians in identifying subtle metabolic patterns associated with different MMA subtypes, facilitating personalized treatment planning
- The increasing integration of telemedicine platforms into rare disease management further supports continuous monitoring of patients with MMA, ensuring timely adjustment of dietary or pharmacological therapies. Digital health solutions are bridging the gap between metabolic specialists and patients, particularly in regions with limited access to expert care
- Furthermore, pharmaceutical research into gene therapy and enzyme replacement therapies (ERT) is accelerating. Companies and research institutions are exploring adeno-associated virus (AAV)–based gene transfer and mRNA-based therapies to correct defective metabolic pathways in MMA
- This trend toward precision medicine and genetic intervention is fundamentally reshaping the MMA treatment landscape, promising not only symptomatic relief but also long-term metabolic correction and improved quality of life for affected individuals
Methylmalonic Acidemia Market Dynamics
Driver
Growing Prevalence of Inherited Metabolic Disorders and Expanding Diagnostic Capabilities
- The rising incidence of inherited metabolic disorders, coupled with growing awareness and screening initiatives, is a significant driver fueling the Methylmalonic Acidemia market growth. Increasing recognition of MMA in newborn screening programs across North America, Europe, and Asia-Pacific has led to early identification and management of affected infants
- For instance, in May 2023, the U.S. Centers for Disease Control and Prevention (CDC) expanded its Recommended Uniform Screening Panel (RUSP) to include more comprehensive metabolic testing, enhancing detection rates of MMA and related organic acidemias
- Pharmaceutical advancements have introduced novel treatment approaches, including cobalamin supplementation, liver transplantation, and emerging enzyme replacement therapies, which have improved patient survival rates and clinical outcomes
- Furthermore, research collaborations between biotechnology companies and academic centers are accelerating therapeutic discovery for rare metabolic disorders. For instance, in 2024, Moderna initiated preclinical studies using mRNA-based technology to restore methylmalonyl-CoA mutase (MUT) enzyme activity in MMA models, marking a major step toward curative therapies
- Increasing patient registries, advocacy initiatives, and data-sharing networks are enhancing research efficiency and supporting the identification of genotype–phenotype correlations, leading to more targeted interventions
- These developments collectively strengthen the diagnostic and therapeutic landscape, fueling global growth in the MMA market and paving the way for precision-based care solutions
Restraint/Challenge
Limited Treatment Options, High Costs, and Diagnostic Delays
- Despite major advancements, the methylmalonic acidemia market continues to face challenges related to limited curative treatment options, high costs of gene therapies, and delays in diagnosis, especially in developing regions
- For instance, the cost of advanced gene therapy and liver transplantation procedures can exceed hundreds of thousands of dollars, making access difficult for many patients, particularly where healthcare reimbursement is limited
- Diagnostic delays are another significant concern due to the rarity and symptom overlap of MMA with other metabolic conditions. Misdiagnosis or late diagnosis can result in irreversible neurological and organ damage, emphasizing the need for improved physician awareness and rapid testing infrastructure
- Availability of specialized diagnostic laboratories and metabolic centers remains uneven across regions, further limiting access to timely care. This regional disparity leads to inconsistent patient outcomes and underreporting of MMA prevalence
- In addition, the small patient population creates financial challenges for pharmaceutical companies in conducting large-scale clinical trials and sustaining long-term drug development efforts. For instance, several investigational enzyme replacement therapies have faced funding obstacles during advanced clinical phases
- High treatment costs, combined with limited reimbursement pathways and resource constraints in healthcare systems, pose a major obstacle to global adoption
- Overcoming these challenges will require collaborative initiatives between governments, private healthcare sectors, and research foundations to establish newborn screening programs, subsidize advanced treatments, and enhance global awareness of methylmalonic acidemia management
Methylmalonic Acidemia Market Scope
The market is segmented on the basis of symptoms and forms.
- By Symptoms
On the basis of symptoms, the global Methylmalonic Acidemia market is segmented into acidosis, ketosis, hyperammonemia, hypoglycemia, hyperglycemia, and neutropenia. The acidosis segment dominated the largest market revenue share of 39.8% in 2024, primarily due to its high prevalence among newly diagnosed patients and its critical role as a key diagnostic indicator of metabolic dysfunction. Acidosis often necessitates immediate clinical intervention, increasing the demand for advanced diagnostic tools and therapeutic approaches. Furthermore, strong focus by healthcare professionals on monitoring blood pH and lactate levels drives consistent diagnostic testing volumes, supporting revenue generation. The segment’s dominance is reinforced by growing awareness among clinicians and the availability of targeted metabolic correction therapies addressing acidosis-related complications.
The hyperammonemia segment is anticipated to witness the fastest growth rate of 18.9% CAGR from 2025 to 2032, fueled by the increasing recognition of ammonia toxicity in infants and children suffering from Methylmalonic Acidemia. Continuous advancements in ammonia-scavenging drugs and diagnostic assays are improving early detection and management of the condition. Moreover, growing research investments into novel therapeutic approaches targeting urea cycle dysfunction and mitochondrial metabolism are accelerating market demand. Enhanced genetic screening programs and newborn screening initiatives worldwide are also contributing to early diagnosis and expanding patient treatment populations.
- By Forms
On the basis of forms, the global Methylmalonic Acidemia market is segmented into isolated methylmalonic acidurias and combined methylmalonic aciduria and homocystinuria. The isolated methylmalonic acidurias segment held the largest market revenue share of 56.3% in 2024, driven by the comparatively higher incidence rate and established diagnostic and therapeutic frameworks supporting its management. Patients with isolated forms typically require lifelong vitamin B12 supplementation and dietary modifications, sustaining demand for targeted treatment options and monitoring systems. In addition, increasing awareness of genetic mutations, such as MUT and MMAA, associated with isolated forms is promoting genetic testing adoption, further expanding diagnostic reach.
The combined methylmalonic aciduria and homocystinuria segment is projected to witness the fastest CAGR of 20.4% from 2025 to 2032, propelled by growing research interest in combined metabolic disorders and the development of novel enzyme replacement and gene therapy-based treatments. This form presents more complex biochemical pathways, attracting higher R&D focus from biopharmaceutical companies aiming to deliver curative approaches. Advances in newborn screening technologies and the integration of multi-omics diagnostics are also facilitating earlier identification of combined cases, contributing to rapid growth in this segment.
Methylmalonic Acidemia Market Regional Analysis
- North America dominated the methylmalonic acidemia (MMA) market with the largest revenue share of 41.5% in 2024, supported by advanced genetic research infrastructure, strong funding for rare disease studies, and the presence of leading biotechnology and pharmaceutical companies focusing on metabolic disorders
- The region benefits from early adoption of gene and mRNA-based therapies, advanced diagnostic technologies, and a well-established network of metabolic disease specialists and treatment centers. Government initiatives promoting rare disease awareness and early genetic screening further strengthen market expansion
- In addition, the growing prevalence of inherited metabolic disorders and ongoing clinical trials for MMA therapies drive continuous innovation and demand for effective treatment options
U.S. Methylmalonic Acidemia Market Insight
The U.S. methylmalonic acidemia (MMA) market captured the largest revenue share of 82% in 2024 within North America, driven by strong investments in genetic research, a high concentration of clinical trials, and the presence of key players such as Moderna, BridgeBio, and Selecta Biosciences. The U.S. has a robust healthcare infrastructure that facilitates early diagnosis, newborn screening programs, and access to novel therapies, including gene therapy and enzyme replacement approaches. Furthermore, strong collaborations between biotechnology companies and federal agencies such as the NIH and FDA are accelerating the approval of next-generation treatments. The combination of government support, precision medicine initiatives, and increased awareness of metabolic disorders continues to propel the U.S. market forward.
Europe Methylmalonic Acidemia Market Insight
The Europe methylmalonic acidemia (MMA) market is projected to expand at a significant CAGR throughout the forecast period, driven by the growing implementation of national newborn screening programs and advancements in genomic research. Europe’s regulatory bodies have been actively supporting orphan drug designations and clinical development for rare metabolic disorders. The rising incidence of inherited metabolic conditions, coupled with strong public health initiatives for early genetic diagnosis, is driving market demand. Moreover, leading biotechnology clusters in Germany, France, and the U.K. are playing a crucial role in developing enzyme replacement and gene-based therapies, while strong collaboration between academic institutions and biotech firms fosters innovation.
U.K. Methylmalonic Acidemia Market Insight
The U.K. methylmalonic acidemia (MMA) market is anticipated to grow at a notable CAGR during the forecast period, supported by increased government funding for rare disease research and early diagnosis initiatives under the NHS Genomic Medicine Service. The U.K. is home to advanced genomic sequencing facilities that enhance the identification of MMA-related mutations and support the development of personalized treatment options. Additionally, collaborations between academic institutions such as the University of Cambridge and leading biotech firms have accelerated the understanding of MMA pathophysiology, further strengthening the country’s therapeutic research landscape.
Germany Methylmalonic Acidemia Market Insight
The Germany methylmalonic acidemia (MMA) market expected to witness strong growth at a substantial CAGR, supported by its advanced biotechnology ecosystem, strong healthcare infrastructure, and increasing focus on rare disease therapeutics. The German government’s support for genomic medicine and translational research has enabled early-stage clinical trials targeting MMA. Germany’s robust presence of academic research institutions, combined with investments in precision medicine and biopharmaceutical innovation, positions it as a key European contributor to the development of enzyme replacement and gene therapy for MMA.
Asia-Pacific Methylmalonic Acidemia Market Insight
The Asia-Pacific methylmalonic acidemia (MMA) market is projected to be the fastest-growing region, registering a high CAGR from 2025 to 2032, driven by improving healthcare infrastructure, rising genetic testing capabilities, and expanding awareness of rare diseases. Countries such as China, Japan, and India are significantly investing in genomic research and newborn screening programs for early detection of metabolic disorders. Increasing collaborations between international biotech firms and regional hospitals are enhancing access to advanced diagnostics and emerging therapies. Furthermore, supportive government initiatives, such as China’s Rare Disease Catalog and Japan’s Orphan Drug Designation framework, are contributing to rapid market expansion.
Japan Methylmalonic Acidemia Market Insight
The Japan methylmalonic acidemia (MMA) market is experiencing steady growth, fueled by the country’s strong emphasis on medical innovation, early screening for rare disorders, and increasing adoption of genomic sequencing technologies. Japan’s Ministry of Health, Labour and Welfare (MHLW) actively supports rare disease treatment research and provides funding for orphan drug development. The growing collaboration between Japanese universities and global biotech firms is leading to new advancements in gene therapy and enzyme replacement research for MMA. Moreover, the country’s aging population and focus on precision medicine continue to boost the demand for genetic testing and early intervention programs.
China Methylmalonic Acidemia Market Insight
The China methylmalonic acidemia (MMA) market accounted for the largest revenue share in Asia-Pacific in 2024, driven by rapid advancements in biotechnology, expansion of genetic testing facilities, and strong government initiatives for rare disease management. The Chinese government’s inclusion of MMA in its national rare disease catalog has accelerated diagnostic efforts and public awareness. Domestic biotech firms are increasingly investing in gene therapy and enzyme production, supported by partnerships with global research organizations. Additionally, China’s growing middle class and healthcare reforms aimed at improving access to specialized care are fostering early diagnosis and treatment adoption, positioning the country as a key player in the Asia-Pacific MMA landscape
Methylmalonic Acidemia Market Share
The Methylmalonic Acidemia industry is primarily led by well-established companies, including:
- Moderna, Inc. (U.S.)
- Recordati Rare Diseases Inc. (U.S.)
- Orphan Technologies Ltd. (Switzerland)
- GeneDx, Inc. (U.S.)
- Agios Pharmaceuticals, Inc. (U.S.)
- Eurofins Scientific (Luxembourg)
- PerkinElmer Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Illumina, Inc. (U.S.)
- Bluebird Bio, Inc. (U.S.)
- Promega Corporation (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Sanofi (France)
- Bruker Corporation (U.S.)
Latest Developments in Global Methylmalonic Acidemia Market
- In August 2022, BridgeBio Pharma, Inc. announced the dosing of the first patient in a Phase 1 trial of its investigational drug BBP-671, designed to treat methylmalonic acidemia and related metabolic disorders by modulating CoA metabolism. This milestone represented a major advancement toward developing a disease-modifying therapy for MMA patients
- In May 2023, Selecta Biosciences, Inc. revealed at the ASGCT annual meeting that its first-in-human clinical trial of gene therapy MMA-101, combined with its proprietary ImmTOR immune tolerance platform, had begun dosing in patients with MMA due to MMUT deficiency. The development marked a key step forward in reducing immune complications in gene therapy
- In June 2024, Moderna, Inc. disclosed that its investigational therapeutic mRNA-3705 for isolated MMA (MUT deficiency) had been selected by the U.S. Food & Drug Administration (FDA) for the START pilot program, highlighting mRNA-based treatment as a promising new class of therapy for rare metabolic disorders
- In September 2024, Genes pire Biotech announced the completion of a US $52 million Series B funding round to accelerate development of its lentiviral gene therapy program GENE202 for pediatric MMA. This funding underscored growing investor confidence in gene therapy as a curative solution for rare diseases
- In March 2025, the National Center for Advancing Translational Sciences (NCATS) and the National Human Genome Research Institute (NHGRI) included MMA-101 for methylmalonic acidemia in their PaVe-GT (Platform Vector Gene Therapy) initiative, with a clinical trial expected to commence in late 2025. This initiative reflected growing institutional support for accelerating gene-based treatments for rare diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.