Global Miniaturized Elisa Devices Market
Market Size in USD Million
CAGR :
% 
 USD
311.29 Million
USD
787.42 Million
2025
2033
USD
311.29 Million
USD
787.42 Million
2025
2033
| 2026 –2033 | |
| USD 311.29 Million | |
| USD 787.42 Million | |
|
|
|
|
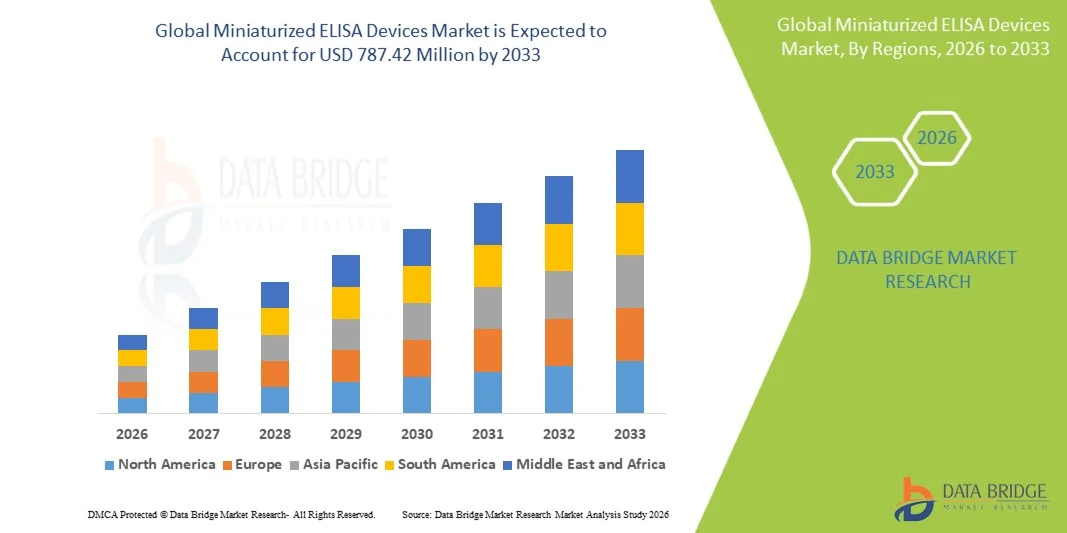
Miniaturized ELISA Devices Market Size
- The global miniaturized ELISA devices market size was valued at USD 311.29 million in 2025 and is expected to reach USD 787.42 million by 2033, at a CAGR of 12.30% during the forecast period
- The market growth is largely fueled by the growing adoption of compact and automated immunoassay devices across diagnostics and research, increasing demand for point‑of‑care solutions, and technological innovations in miniaturization, fluid handling and multiplexing in ELISA formats
- Furthermore, rising needs for high‑throughput, cost‑efficient, and user‑friendly platforms in both clinical and laboratory settings are positioning miniaturized ELISA devices as preferred analytical tools, thereby significantly boosting the industry’s expansion
Miniaturized ELISA Devices Market Analysis
- Miniaturized ELISA devices, offering compact or portable immunoassay platforms for diagnostics, research, and field‑testing, are increasingly vital components of modern clinical and laboratory workflows in both healthcare and research settings due to their rapid results, low reagent consumption, and potential for point‑of‑care or decentralized testing
- The escalating demand for miniaturized ELISA devices is primarily fueled by technological advancements in microfluidics and lab‑on‑chip systems, growing emphasis on decentralized and rapid diagnostics, and a rising preference for cost‑efficient, high‑throughput, and user‑friendly immunoassay solutions
- North America dominated the miniaturized ELISA devices market with the largest revenue share of 38.9% in 2025, characterized by advanced healthcare infrastructure, high adoption of automated diagnostic technologies, and a strong presence of key industry players
- Asia‑Pacific is expected to be the fastest‑growing region in the miniaturized ELISA devices market during the forecast period due to increasing healthcare access, rising R&D investment, and growing adoption of point‑of‑care diagnostic technologies in emerging economies
- The point‑of‑care (POC) miniaturized ELISA device segment dominated the miniaturized ELISA devices market with a leading market share of 50.2% in 2025, driven by their established reputation for rapid testing, ease of use, and suitability for decentralized laboratory and field applications
Report Scope and Miniaturized ELISA Devices Market Segmentation
|
Attributes |
Miniaturized ELISA Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Miniaturized ELISA Devices Market Trends
“Integration of Microfluidics and IoT for Enhanced Diagnostics”
- A significant and accelerating trend in the global miniaturized ELISA devices market is the integration of microfluidics, lab-on-chip technology, and IoT-enabled data connectivity, enhancing the speed, accuracy, and portability of immunoassay testing
- For instance, the Abingdon MicroELISA platform integrates microfluidic cartridges with cloud-based result tracking, enabling real-time monitoring and analysis for decentralized clinical and research applications
- IoT-enabled miniaturized ELISA devices allow automatic data capture, remote monitoring of assay results, and real-time quality control alerts, facilitating faster decision-making in diagnostics and research workflows
- The seamless connection of these devices with digital laboratory management systems and remote platforms enables centralized control, monitoring multiple assays across locations and automating routine laboratory tasks
- This trend towards highly automated, connected, and miniaturized immunoassay platforms is reshaping user expectations for speed, portability, and convenience in diagnostic testing
- The demand for compact, networked ELISA devices is growing rapidly across clinical, research, and field-testing applications, as users increasingly prioritize rapid, reliable, and data-integrated testing solutions
Miniaturized ELISA Devices Market Dynamics
Driver
“Rising Demand for Rapid, Decentralized Diagnostics”
- The increasing need for fast, accurate, and decentralized diagnostic testing in both clinical and research environments is a significant driver for the growing adoption of miniaturized ELISA devices
- For instance, in March 2025, Abcam launched a portable ELISA platform designed for point-of-care immunoassays, facilitating rapid biomarker detection in decentralized laboratory settings
- As healthcare providers and research institutes seek to reduce turnaround times and improve throughput, miniaturized ELISA platforms offer high efficiency, automation, and low sample/reagent requirements
- Furthermore, the expansion of point-of-care diagnostics and field-based testing is creating demand for compact, portable immunoassay solutions capable of operating outside centralized laboratories
- Increasing funding and investment in biotechnology and clinical diagnostics is accelerating the development and deployment of advanced miniaturized ELISA platforms
- Rising prevalence of infectious diseases and chronic illnesses is driving demand for rapid biomarker detection, fueling the adoption of portable and automated ELISA systems
- The ability to perform rapid, high-accuracy testing with minimal technical expertise and integrate results with digital data management systems is a key factor propelling market adoption in both clinical and research applications
Restraint/Challenge
“High Device Costs and Technical Complexity”
- The relatively high cost of miniaturized ELISA devices, especially those with multiplexing, automation, or IoT capabilities, poses a challenge to widespread adoption in cost-sensitive laboratories and emerging markets
- For instance, advanced microfluidic ELISA systems from companies such as Abingdon or BioTek often require significant upfront investment and specialized consumables, limiting accessibility for smaller research labs
- In addition, operating these devices may require technical training or integration with laboratory management software, which can hinder adoption in low-resource or decentralized settings
- Limited standardization of miniaturized ELISA platforms across different manufacturers can complicate integration with existing laboratory workflows and software systems
- Maintenance and calibration of sensitive microfluidic or multiplexed ELISA devices require specialized technical support, adding operational complexity
- Addressing affordability and providing user-friendly interfaces, along with adequate training and support, is crucial for expanding the market reach of miniaturized ELISA solutions
- Overcoming these challenges through cost reduction, simplified operation, and scalable platform designs will be vital for sustaining market growth and broader penetration across diverse clinical and research environments
Miniaturized ELISA Devices Market Scope
The market is segmented on the basis of device type, technology, application, and end user.
- By Device Type
On the basis of device type, the miniaturized ELISA devices market is segmented into manual miniaturized ELISA devices, fully‑automated miniaturized ELISA platforms, point‑of‑care (POC) miniaturized ELISA devices, consumables & accessories, and software & services associated with miniaturized ELISA platforms. The point‑of‑care (POC) miniaturized ELISA devices segment dominated the miniaturized ELISA devices market with a leading market share of 50.2% in 2025, driven by its portability, rapid results, and suitability for decentralized testing in clinics, mobile labs, and field applications. Healthcare providers and research institutes often prioritize POC devices for quick turnaround times, low sample/reagent requirements, and ease of use outside traditional laboratory settings. Technological advances in microfluidics, IoT-enabled connectivity, and simplified interfaces have further strengthened adoption. In addition, the segment benefits from the rising trend of personalized medicine, home testing, and rapid biomarker screening. The high demand for flexible, compact, and cost-efficient platforms reinforces its dominance in both developed and emerging markets.
The fully‑automated miniaturized ELISA platforms segment is anticipated to witness the fastest growth from 2026 to 2033, fueled by increasing adoption in large clinical laboratories, hospitals, and pharmaceutical/biotechnology R&D settings. Fully-automated platforms provide high throughput, reproducibility, and integration with laboratory information systems, making them ideal for handling large sample volumes. Automation reduces hands-on time and human error, improving efficiency in high-demand testing environments. Advances in multiplexing, AI-assisted assay optimization, and digital data management are accelerating adoption in research and clinical labs globally. The ability to handle complex and large-scale immunoassays positions this segment for rapid growth over the forecast period.
- By Technology
On the basis of technology, the miniaturized ELISA devices market is segmented into colourimetric ELISA, fluorescence-based ELISA, chemiluminescent ELISA, multiplex ELISA, and microfluidic ELISA. The chemiluminescent ELISA segment dominated the market with the largest market revenue share in 2025, owing to its high sensitivity, wide dynamic range, and suitability for clinical diagnostics and biomarker quantification. Laboratories often prefer chemiluminescent detection for low-abundance analytes, multiplexed assays, and high-throughput workflows. The segment’s dominance is reinforced by its integration with fully-automated platforms, enabling accurate and reproducible results across multiple applications. Chemiluminescent ELISA also supports standardized protocols, regulatory compliance, and high analytical performance, making it a trusted choice for hospitals and research institutions. Its adoption is especially high in developed regions where investment in advanced diagnostics is substantial. The segment continues to grow as manufacturers enhance reagent chemistry, improve detection limits, and integrate digital readouts for seamless data management.
The microfluidic/lab-on-chip ELISA segment is expected to witness the fastest growth from 2026 to 2033, driven by miniaturization trends, reduced reagent/sample volumes, and portability. Microfluidic platforms enable rapid assay execution, multiplexing capabilities, and decentralized testing, which are increasingly in demand in emerging markets and point-of-care settings. Integration with IoT and AI-assisted data analysis further enhances usability and performance. In addition, technological advances such as 3D printing of microchannels, low-cost consumables, and simplified user interfaces support faster adoption. The growth is also propelled by demand in field-testing, mobile labs, and research applications requiring compact, cost-efficient solutions. As manufacturing and standardization improve, adoption rates are expected to accelerate globally.
- By Application
On the basis of application, the miniaturized ELISA devices market is segmented into infectious disease diagnostics, cancer biomarker testing, autoimmune disorders, hormone testing, biotech R&D, veterinary diagnostics, and food safety & environmental monitoring. The infectious disease diagnostics segment dominated the market with the largest market revenue share in 2025, owing to the widespread use of ELISA for antigen and antibody detection. Clinical laboratories and hospitals prioritize this application due to the high volume of tests, regulatory support, and standardized protocols. The segment benefits from ongoing public health initiatives, epidemic preparedness programs, and the need for rapid and reliable testing. The high demand for accurate diagnostics in both developed and emerging markets reinforces its dominance. In addition, advancements in fully-automated ELISA platforms and chemiluminescent detection improve throughput and reproducibility, supporting continuous growth. The segment remains vital due to its role in disease surveillance, outbreak management, and global health monitoring.
The cancer biomarker testing segment is anticipated to witness the fastest growth from 2026 to 2033, fueled by the rising prevalence of cancer and increasing investment in personalized medicine and biomarker research. Miniaturized ELISA devices are highly suitable for multiplex assays, enabling simultaneous detection of multiple biomarkers in small sample volumes. Pharmaceutical and biotechnology R&D programs are driving demand for rapid, sensitive, and portable platforms. In addition, the trend toward decentralized testing and translational research increases adoption in both academic and clinical research settings. Technological innovations in multiplexing, AI-assisted analysis, and microfluidics further accelerate growth. The segment’s expansion is supported by ongoing funding, research collaborations, and the need for early disease detection and treatment monitoring.
- By End User
On the basis of end user, the miniaturized ELISA devices market is segmented into hospitals & clinical diagnostic laboratories, research institutes, pharmaceutical & biotechnology companies, field labs, and food testing laboratories. The hospitals & clinical diagnostic laboratories segment dominated the market with the largest market revenue share in 2025, owing to high adoption of fully-automated platforms, validated protocols, and integration with laboratory information systems. These facilities demand high throughput, reproducibility, and regulatory compliance, making them key buyers for miniaturized ELISA devices. Hospitals and large diagnostic labs are equipped to handle large sample volumes and diverse assays, reinforcing segment dominance. The segment benefits from reimbursement frameworks, established procurement channels, and strong purchasing power. In addition, increasing adoption of point-of-care testing within hospital networks supports market growth. Manufacturers target this segment with advanced, validated, and automated solutions to meet institutional requirements.
The research institutes segment is anticipated to witness the fastest growth from 2026 to 2033, driven by rising investment in biomarker discovery, drug development, immunology, and translational medicine. Miniaturized ELISA devices are attractive due to portability, cost efficiency, small sample volume usage, and flexible assay configurations. Academic and research labs prioritize multiplexed, automated, and microfluidic platforms for rapid experimentation and high-throughput screening. The segment benefits from collaborations with pharmaceutical companies, grants, and increasing research funding globally. In addition, the demand for decentralized, flexible, and easy-to-operate ELISA solutions accelerates adoption in this end-user segment.
Miniaturized ELISA Devices Market Regional Analysis
- North America dominated the miniaturized ELISA devices market with the largest revenue share of 38.9% in 2025, characterized by advanced healthcare infrastructure, high adoption of automated diagnostic technologies, and a strong presence of key industry players
- The region benefits from strong investments in microfluidics, bioassay miniaturization, and precision diagnostics, with leading diagnostic firms and academic institutions spearheading technological advancements
- Growing demand for rapid, decentralized testing solutions in hospitals, clinics, and research laboratories, coupled with favorable regulatory support for diagnostic innovations, has significantly strengthened market growth in the U.S. and Canada
U.S. Miniaturized ELISA Devices Market Insight
The U.S. miniaturized ELISA devices market captured the largest revenue share of 78.6% in 2025 within North America, driven by the strong presence of leading diagnostic and biotechnology companies and the rapid adoption of advanced analytical technologies. The country’s well-established healthcare infrastructure and significant funding for biomedical research continue to accelerate the adoption of automated and microfluidic ELISA platforms. Increasing demand for decentralized and point-of-care testing across hospitals, clinics, and research institutions is further propelling market growth. Moreover, ongoing innovation in microchip-based ELISA, coupled with regulatory support for digital diagnostics and AI-driven assay analysis, reinforces the U.S. as a key global hub for miniaturized ELISA device development and commercialization.
Europe Miniaturized ELISA Devices Market Insight
The Europe miniaturized ELISA devices market is projected to expand at a substantial CAGR throughout the forecast period, driven by the region’s strong emphasis on diagnostic accuracy, technological innovation, and stringent regulatory compliance. Increasing investment in healthcare modernization, coupled with the rising prevalence of infectious and chronic diseases, is fostering the adoption of advanced immunoassay technologies. European laboratories and diagnostic centers are rapidly integrating automated and portable ELISA systems for faster, more reliable testing. The region is also seeing a growing preference for sustainable, energy-efficient laboratory devices that align with EU environmental standards. Research collaborations and government funding initiatives are expected to sustain steady market growth across clinical and academic sectors.
U.K. Miniaturized ELISA Devices Market Insight
The U.K. miniaturized ELISA devices market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by expanding biomedical research capabilities and the growing focus on rapid, accurate diagnostic solutions. The country’s National Health Service (NHS) and private healthcare providers are increasingly adopting automated ELISA platforms to enhance testing throughput and efficiency. Rising investments in life sciences innovation, coupled with the U.K.’s robust academic ecosystem, are accelerating the development of microfluidic and AI-assisted ELISA systems. In addition, post-Brexit initiatives encouraging domestic manufacturing and R&D are supporting the market’s upward trajectory, particularly across clinical, research, and public health applications.
Germany Miniaturized ELISA Devices Market Insight
The Germany miniaturized ELISA devices market is expected to expand at a considerable CAGR during the forecast period, driven by the country’s advanced healthcare infrastructure and emphasis on laboratory automation and precision diagnostics. Germany’s strong presence of biotechnology firms and academic research institutions continues to drive innovation in miniaturized and multiplex ELISA systems. The growing demand for portable diagnostic platforms in decentralized and hospital-based settings, combined with regulatory support for high-quality in-vitro diagnostics, underpins market expansion. Moreover, the country’s focus on sustainability and technological reliability aligns with the adoption of energy-efficient, compact diagnostic instruments.
Asia-Pacific Miniaturized ELISA Devices Market Insight
The Asia-Pacific miniaturized ELISA devices market is poised to grow at the fastest CAGR of 23.5% during the forecast period of 2026 to 2033, driven by rising healthcare expenditure, expanding diagnostic capacity, and rapid technological advancement in countries such as China, Japan, and India. Increasing demand for portable, affordable testing solutions is fueling adoption across hospitals, clinics, and field laboratories. Government initiatives promoting healthcare digitalization and point-of-care testing are further accelerating growth. As the region strengthens its position as a global manufacturing hub for diagnostic instruments and consumables, greater affordability and accessibility are extending market reach across both urban and rural populations.
Japan Miniaturized ELISA Devices Market Insight
The Japan miniaturized ELISA devices market is gaining momentum due to the country’s strong culture of technological innovation and focus on precision diagnostics. High demand for rapid, automated testing in hospitals and research institutes is driving adoption of microfluidic and multiplex ELISA systems. Integration of ELISA devices with smart data management tools and IoT platforms is improving laboratory efficiency and clinical decision-making. Japan’s aging population and emphasis on preventive healthcare are further boosting demand for compact, reliable diagnostic devices. In addition, the nation’s commitment to healthcare automation and digital transformation is positioning it as a key market for next-generation ELISA technologies.
India Miniaturized ELISA Devices Market Insight
The India miniaturized ELISA devices market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rapid healthcare infrastructure expansion, increasing disease awareness, and strong growth in diagnostic testing demand. India’s vast network of hospitals, diagnostic centers, and emerging biotech startups are adopting cost-efficient miniaturized ELISA platforms for infectious disease and biomarker testing. Government programs such as Digital India and Make in India are encouraging local innovation and domestic production of diagnostic technologies. In addition, the surge in private healthcare investment and the expansion of decentralized testing facilities are accelerating market growth. The combination of affordability, accessibility, and growing research capacity reinforces India’s role as a key growth driver in the regional market.
Miniaturized ELISA Devices Market Share
The Miniaturized ELISA Devices industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Merck KGaA (Germany)
- Abcam Limited. (U.K.)
- Bio-Techne Corporation (U.S.)
- PerkinElmer (U.S.)
- QIAGEN (Netherlands)
- Siemens Healthineers AG (Germany)
- F. Hoffmann-La Roche Ltd (Switzerland)
- BD (U.S.)
- DiaSorin S.p.A. (Italy)
- Enzo Biochem, Inc. (U.S.)
- HyTest Ltd (Finland)
- R-Biopharm AG (Germany)
- Randox Laboratories Ltd (U.K.)
- Trinity Biotech plc (Ireland)
- SEKISUI Diagnostics (Japan)
- BioVendor (Czech Republic)
- GenScript (China)
- ORGENTEC (Germany)
What are the Recent Developments in Global Miniaturized ELISA Devices Market?
- In March 2025, researchers unveiled a miniaturized, automated, and cost-effective ELISA device featuring dimensions of 24 cm × 19 cm × 14 cm and weighing less than 3 kg; the hardware cost is estimated at approximately USD 1,200, with a per-test cost of under USD 10, making it suitable for point-of-care applications in resource-limited regions
- In December 2023, LANSING (food diagnostics provider) launched the Veratox VIP assay, an enhanced ELISA capable of detecting walnut allergens down to 0.15 ppm, advancing food-safety applications. It highlights expanding applications of ELISA (including miniaturised versions) into food safety and environmental monitoring
- In June 2023, a hand-held plug-and-play microfluidic liquid-handling automation platform was described, featuring a “clamshell-style” cartridge socket designed for miniaturized immunoassay workflows. This platform allows automated liquid‐handling in a compact form factor, enabling immunoassay operations outside conventional lab benches
- In February 2022, researchers developed “advection-enhanced kinetics in microtiter plates” to improve immunoassay sensitivity and speed by integrating microscale flows into conventional ELISA plate formats. This innovation enhances traditional ELISA workflows by incorporating micro-fluidic flow dynamics, reducing incubation times and improving assay sensitivity
- In October 2021, a study described an automated ELISA on-chip for detecting anti-SARS-CoV-2 antibodies in serum, using microfluidics and smartphone camera analysis, showing no statistically significant difference compared to traditional plate-ELISA
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.