Global Minimally Invasive Neurosurgical Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
2.00 Billion
USD
3.19 Billion
2024
2032
USD
2.00 Billion
USD
3.19 Billion
2024
2032
| 2025 –2032 | |
| USD 2.00 Billion | |
| USD 3.19 Billion | |
|
|
|
|
Minimally Invasive Neurosurgical Devices Market Analysis
The minimally invasive neurosurgical devices market has seen substantial growth, driven by technological advancements and increasing demand for less invasive procedures. Minimally invasive techniques are favored for their benefits, including reduced recovery times, decreased surgical risks, and shorter hospital stays. Advancements in medical technology such as enhanced imaging systems, robotic-assisted surgical tools, and improved navigation and visualization devices have revolutionized the field, enabling more precise procedures and better patient outcomes. Companies are investing heavily in developing cutting-edge devices, such as advanced endoscopy systems, neuronavigation tools, and surgical robots, to address complex neurological conditions with minimal disruption to healthy tissue. Minimally invasive procedures are increasingly used in various surgeries, including spinal, cranial, and neurovascular procedures, due to their effectiveness and lower complication rates compared to traditional open surgery. The market is also supported by a growing focus on patient-centered care and cost-effective solutions that reduce healthcare expenditure. With ongoing innovations and an aging global population prone to neurovascular diseases, the market is projected to continue expanding, presenting opportunities for new entrants and existing players to develop and expand their product portfolios.
Minimally Invasive Neurosurgical Devices Market Size
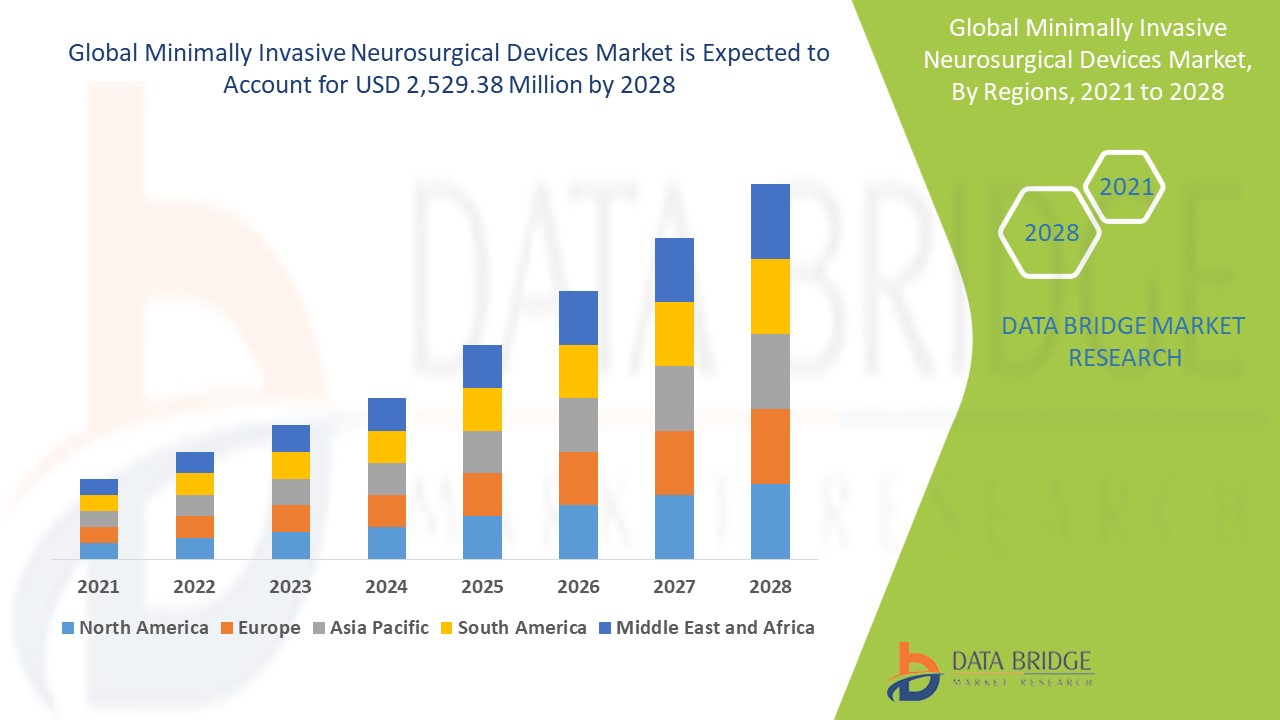
The global minimally invasive neurosurgical devices market size was valued at USD 2.00 billion in 2024 and is projected to reach USD 3.19 billion by 2032, with a CAGR of 6.02% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Minimally Invasive Neurosurgical Devices Market Trends
“Integration of Robotic-Assisted Surgical Systems”
One key trend in the minimally invasive neurosurgical devices market is the integration of robotic-assisted surgical systems, which enhance the precision and efficiency of complex procedures. Robotic systems, such as those developed by Medtronic and Brainlab, enable surgeons to perform delicate operations with greater accuracy, reducing the risk of complications and improving patient outcomes. These systems provide real-time visualization and advanced navigation capabilities, making them invaluable for spinal surgeries and neurovascular procedures. For instance, Medtronic’s Stealth Autoguide system helps neurosurgeons map out surgical plans and guide instruments with pinpoint precision. This trend aligns with the industry's emphasis on minimizing invasiveness, which leads to shorter recovery times and fewer post-surgery complications. The growing adoption of robotic technology reflects an increasing demand for high-precision tools that support better patient care and align with the healthcare sector's push for innovation and improved surgical results.
Report Scope and Minimally Invasive Neurosurgical Devices Market Segmentation
|
Attributes |
Minimally Invasive Neurosurgical Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
General Medical Inc. (U.S.), Stryker (U.S.), Zimmer Biomet (U.S.), B. Braun SE (Germany), Olympus Corporation (Japan), Renishaw plc. (U.K.), Medtronic (Ireland), Danaher (U.S.), ZEISS AG (Germany), KARL STORZ (Germany), Brainlab AG (Germany), Monteris (Canada), INTEGRA LIFESCIENCES (U.S.), Penumbra, Inc. (U.S.), Aesculap, Inc. (Germany), Boston Scientific Corporation (U.S.), Richard Wolf GmbH (Germany), Nico Corporation (U.S.), Soring GmbH (Germany), Vycor Medical Inc. (U.S.), CONMED Corporation (U.S.), Johnson & Johnson Services Inc. (U.S.), and Microbot Medical Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Minimally Invasive Neurosurgical Devices Market Definition
Minimally invasive neurosurgical devices refer to specialized medical instruments and technologies designed to perform surgical procedures on the nervous system with minimal disruption to surrounding tissues. These devices enable neurosurgeons to access and treat areas of the brain, spine, and peripheral nervous system using small incisions or specialized techniques, which reduces the risk of infection, shortens recovery time, and minimizes post-operative pain compared to traditional open surgeries.
Minimally Invasive Neurosurgical Devices Market Dynamics
Drivers
- Growing Prevalence of Neurological Disorders
The growing prevalence of neurological disorders is a significant driver for the minimally invasive neurosurgical devices market, as the rising incidence of conditions such as neurovascular diseases, spinal disorders, and brain-related disorders continues to increase the need for effective surgical treatments. For instance, according to the World Health Organization (WHO), around 50 million people worldwide suffer from epilepsy, a common neurological disorder, and stroke remains one of the leading causes of death globally. In addition, the number of spinal procedures has surged due to factors such as an aging population and sedentary lifestyles, which contribute to conditions such as herniated discs and spinal stenosis. This heightened demand for surgical intervention has spurred the adoption of advanced minimally invasive technologies, as they offer improved patient outcomes, quicker recovery times, and lower risks of complications compared to traditional surgery. As a result, the prevalence of neurological disorders continues to propel market growth, prompting the development and use of innovative surgical solutions.
- Increasing Geriatric Population
The increasing geriatric population is a major driver for the minimally invasive neurosurgical devices market, as older adults are more susceptible to neurological disorders and conditions that require surgical intervention. According to the United Nations, the global population aged 60 years and over is expected to reach 2.1 billion by 2050, up from 1 billion in 2020, highlighting the rapid growth of the elderly demographic. This age group is particularly prone to conditions such as spinal disorders, neurovascular diseases, and degenerative brain conditions that often necessitate surgery. Minimally invasive neurosurgical techniques are particularly appealing for treating the elderly, as they involve less trauma, faster recovery, and reduced risk of complications, which aligns with the medical needs and preferences of this population. Consequently, the expanding elderly population is fueling demand for safer, more efficient surgical solutions, driving growth in the market for minimally invasive neurosurgical devices.
Opportunities
- Increasing Advancements in Technology
Advancements in technology have created significant market opportunities in the minimally invasive neurosurgical devices market, as innovations in imaging systems, robotic-assisted surgical tools, and enhanced navigation and visualization devices have greatly improved the precision and efficiency of these procedures. For instance, advanced neuronavigation systems such as the Medtronic Stealth Autoguide and Brainlab's Curve systems allow neurosurgeons to accurately map out surgical paths and make real-time adjustments, minimizing risk and enhancing patient outcomes. The integration of 3D imaging and augmented reality in surgical planning further enables more precise targeting and reduces the need for large incisions. In addition, robotic surgery, such as the use of the Mazor X Stealth Edition by Medtronic, has introduced greater control and accuracy to complex procedures. These technological advancements drive improved surgical results and expand the scope of procedures that can be performed using minimally invasive techniques, creating significant growth opportunities within the market.
- Rising Government Initiatives and Investments
Government initiatives and investments are creating substantial opportunities in the minimally invasive neurosurgical devices market, as both public and private sectors increase their support for the development and adoption of advanced medical technologies. For instance, countries such as the U.S. have launched programs such as the National Institute of Neurological Disorders and Stroke (NINDS), which funds research and development for innovative neurosurgical solutions. Similarly, European Union grants and partnerships with healthcare institutions encourage the creation of state-of-the-art medical devices, contributing to advancements in surgical techniques and patient care. Private sector investments are also on the rise, with companies such as Medtronic and Brainlab channeling substantial resources into R&D to push the boundaries of minimally invasive procedures. This combined push from both governmental bodies and private enterprises supports a robust innovation cycle, leading to the development of new, more effective neurosurgical devices and driving market growth.
Restraints/Challenges
- High Costs of Minimally Invasive Neurosurgical Devices
High costs present a significant challenge in the minimally invasive neurosurgical devices market, potentially limiting their adoption and accessibility, especially in regions with budget constraints or lower-income healthcare systems. The development and manufacturing of advanced neurosurgical devices involve significant investment in research, materials, precision engineering, and regulatory compliance, which can drive up prices. For instance, high-tech imaging systems and robotic-assisted surgical devices are costly to produce and maintain. In countries with constrained healthcare budgets, hospitals and surgical centers may find it difficult to justify the expense of incorporating these devices, opting instead for more traditional and less costly methods. This can create a gap in access to cutting-edge medical treatments, impacting patient outcomes and overall market growth. The challenge of high costs emphasizes the need for strategies that make advanced devices more affordable, such as partnerships between manufacturers and healthcare providers, public-private funding, or tiered pricing models that can cater to different economic conditions.
- Patient Safety and Complications
Patient safety and complications remain a crucial challenge in the minimally invasive neurosurgical devices market, despite their benefits of shorter recovery times and reduced hospital stays. Although these procedures are designed to minimize disruption to surrounding tissues and lower the risk of complications compared to traditional open surgeries, the potential for adverse outcomes such as infections, bleeding, or damage to surrounding neural structures still exists. For instance, complications from minimally invasive spine surgeries, such as inadvertent nerve injury, can lead to long-term disability and negatively impact patient trust in these advanced techniques. Managing these risks effectively requires continuous innovation in device design, rigorous training for surgeons, and stringent quality control processes, all of which add to the complexity and cost of bringing these devices to market. Failure to ensure high patient safety standards can erode trust, hinder adoption, and slow the growth of the market. This challenge underscores the need for ongoing advancements in device technology, comprehensive surgical training programs, and robust post-operative monitoring to mitigate risks and improve patient outcomes.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Minimally Invasive Neurosurgical Devices Market Scope
The market is segmented on the basis of product, surgery type, and end user. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Surgical Devices
- Monitoring and Visualization Devices
- Endoscopy Devices
Surgery Type
- Endonasal Neurosurgery
- Intracranial Surgery
- Spinal Surgery
- Others
End User
- Hospital
- Clinics
- Medical Institution
- Ambulatory Surgical Center
Minimally Invasive Neurosurgical Devices Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, product, surgery type, and end user as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the minimally invasive neurosurgical devices market, driven by the increasing prevalence of neurovascular diseases and the growing awareness among neurosurgeons and medical professionals about the advantages of using advanced neurology devices. Europe holds the second-largest share in the market, propelled by comparable factors, such as the rising incidence of neurological disorders and the emphasis on modern surgical techniques. Both regions benefit from a strong presence of highly skilled surgeons and healthcare professionals, along with advanced medical infrastructure and well-established healthcare facilities. These factors collectively contribute to their dominance in the global market, showcasing their capacity to adopt and implement innovative surgical solutions effectively.
Asia-Pacific region is becoming an increasingly profitable market for minimally invasive neurosurgical devices from 2025 to 2032, driven by growing awareness about the benefits of these advanced medical devices and a rising population. In addition, government initiatives aimed at enhancing healthcare infrastructure and boosting medical investments are playing a crucial role in supporting market growth. The region's focus on improving medical access and quality of care is encouraging the adoption of innovative neurology technologies. This combination of factors positions Asia-Pacific as a key area for expansion in the minimally invasive neurosurgical devices market over the forecast period.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Minimally Invasive Neurosurgical Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Minimally Invasive Neurosurgical Devices Market Leaders Operating in the Market Are:
- General Medical Inc. (U.S.)
- Stryker (U.S.)
- Zimmer Biomet (U.S.)
- B. Braun SE (Germany)
- Olympus Corporation (Japan)
- Renishaw plc. (U.K.)
- Medtronic (Ireland)
- Danaher (U.S.)
- ZEISS AG (Germany)
- KARL STORZ (Germany)
- Brainlab AG (Germany)
- Monteris (Canada)
- INTEGRA LIFESCIENCES (U.S.)
- Penumbra, Inc. (U.S.)
- Aesculap, Inc. (Germany)
- Boston Scientific Corporation (U.S.)
- Richard Wolf GmbH (Germany)
- Nico Corporation (U.S.)
- Soring GmbH (Germany)
- Vycor Medical Inc. (U.S.)
- CONMED Corporation (U.S.)
- Johnson & Johnson Services Inc. (U.S.)
- Microbot Medical Inc. (U.S.)
Latest Developments in Minimally Invasive Neurosurgical Devices Market
- In April 2024, Dr. Reddy’s launched Nerivio, a drug-free, noninvasive device for migraine management. Supported by the company's M-free patient support program, this device aims to enhance the patient experience with services including onboarding, counseling, device demonstrations, doorstep delivery, flexible payment plans, educational content, and dedicated customer support.
- In January 2023, PENTAX Medical received CE marks for its new premium video processor, the PENTAX Medical INSPIRA, and the i20c video endoscope series. This new processor is compatible with PENTAX Medical's latest endoscope models and introduces new standards for high-quality imaging.
- In August 2023, Orthofix Medical announced the full commercial launch of their 7D Flash navigation system percutaneous module 2.0 in the U.S.. This enhanced version adds new planning features and broadens the utility of the 7D Flash neuronavigation system for minimally invasive spinal surgeries. The 7D system was originally provided by 7D Surgical, which was acquired by SeaSpine in 2021 after the merger between Orthofix and SeaSpine earlier this year.
- In October 2022, scientists from Imperial College London successfully implanted a new bioinspired, flexible, and steerable device into the brains of living animals using a minimally invasive robotic system for the first time. This advanced platform offers high precision and less intrusive procedures and could potentially improve the detection and treatment of diseases in humans if proven safe and effective.
- In June 2022, Boston Scientific entered into an agreement to acquire a majority stake in M.I.Tech, a South Korean manufacturer and distributor of non-vascular metal stents used for endoscopic and urologic procedures. The deal was valued at approximately USD 230 million, with Boston Scientific paying about approximately USD 11 per M.I.Tech share.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.