Global Molecular Diagnostics Market
Market Size in USD Billion
CAGR :
% 
 USD
25.80 Billion
USD
70.05 Billion
2024
2032
USD
25.80 Billion
USD
70.05 Billion
2024
2032
| 2025 –2032 | |
| USD 25.80 Billion | |
| USD 70.05 Billion | |
|
|
|
|
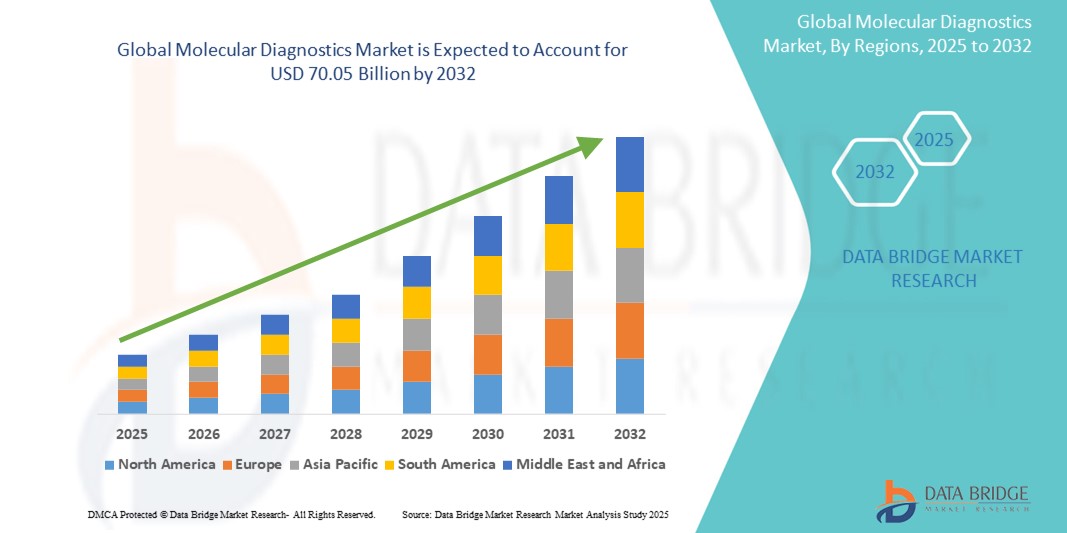
Molecular Diagnostics Market Size
- The global molecular diagnostics market was valued at USD 25.8 billion in 2024 and is expected to reach USD 70.05 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 13.30%, primarily driven by high usage of PCR
- This growth is driven by factors such as accuracy and sensitivity and rapid turnaround time
Molecular Diagnostics Market Analysis
- Molecular diagnostics refers to a field of medical testing that utilizes molecular biology techniques, such as PCR and NGS, to detect and monitor diseases, identify genetic predispositions, and guide targeted therapies, widely applied in healthcare, clinical laboratories, and research settings
- Market growth is driven by the rising prevalence of infectious diseases and chronic conditions, increasing demand for personalized medicine, and the growing adoption of advanced diagnostic technologies such as real-time PCR and next-generation sequencing
- The market is evolving with technological innovations in automated platforms, integration of AI and machine learning for result interpretation, and the development of portable, point-of-care diagnostic devices enhancing speed, accuracy, and accessibility
- For instance, companies such as Roche and Thermo Fisher Scientific are leveraging digital technologies and automation to deliver faster, more precise molecular diagnostic solutions tailored to clinical and laboratory needs
- The molecular diagnostics market is expected to witness sustained growth, supported by ongoing advancements in genomics, expanding healthcare infrastructure, and increased investment in early disease detection and precision medicine initiatives
Report Scope and Molecular Diagnostics Market Segmentation
|
Attributes |
Molecular Diagnostics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Molecular Diagnostics Market Trends
“Increasing Adoption of Liquid Biopsy Techniques”
- One prominent trend in the global molecular diagnostics market is the increasing adoption of liquid biopsy techniques
- This trend is driven by the growing demand for non-invasive diagnostic methods, the need for real-time monitoring of disease progression, and the rising emphasis on personalized treatment strategies, particularly in oncology
- For instance, companies such as Guardant Health and Biocept are advancing liquid biopsy technologies that analyze circulating tumor DNA (ctDNA) and other biomarkers to enable early cancer detection and therapy selection
- The continued shift toward minimally invasive procedures, along with technological improvements in biomarker detection and genomic analysis, is accelerating the integration of liquid biopsy into routine clinical practice
- As healthcare systems focus on precision medicine, early diagnosis, and improved patient outcomes, liquid biopsy is expected to play a transformative role in expanding access to timely, accurate, and personalized diagnostics across various disease areas
Molecular Diagnostics Market Dynamics
Driver
“Increasing Elderly Population”
- The growing elderly population is a key driver of growth in the global molecular diagnostics market
- This demographic shift is contributing to a higher incidence of age-related diseases such as cancer, cardiovascular disorders, and neurodegenerative conditions, driving the demand for early and accurate diagnostic solutions
- As the aging population requires more frequent health monitoring and personalized care, molecular diagnostics offers non-invasive, rapid, and precise testing options suited for chronic disease management and preventive healthcare
- Benefits such as early detection, targeted therapy guidance, and real-time disease tracking are making molecular diagnostics an essential tool in elderly care strategies across healthcare systems
- Diagnostic solution providers are responding by developing user-friendly, minimally invasive platforms tailored to the needs of aging patients and expanding access through home-based testing and telehealth integration
For instance,
- Roche and Abbott are advancing molecular testing tools for early detection of cancer and infectious diseases, specifically addressing the diagnostic needs of the elderly
- Thermo Fisher Scientific is enhancing its genomic platforms to support personalized diagnostics for age-related genetic risks
- As the global population continues to age and healthcare priorities shift toward prevention and personalized care, the role of molecular diagnostics is expected to expand significantly in supporting long-term health outcomes for older adults
Opportunity
“Increasing Demand for Molecular Diagnostics in Point-of-Care Setting”
- The growing demand for molecular diagnostics in point-of-care (POC) settings presents a significant opportunity in the molecular diagnostics market. By enabling rapid, on-site testing, POC diagnostics are enhancing accessibility, speed, and clinical decision-making in real-time
- Advancements in portable technologies, user-friendly assay formats, and integrated sample-to-result systems are empowering healthcare providers to deliver immediate diagnostic insights in settings such as clinics, emergency rooms, and remote locations
- Features such as compact design, minimal sample requirements, and automated workflows are being leveraged to support quicker diagnosis and treatment initiation, particularly in infectious disease management and chronic care monitoring
For instance,
- Cepheid’s GeneXpert and Abbott’s ID NOW platforms are widely used in decentralized healthcare environments for fast, accurate molecular testing
- bioMérieux is developing compact molecular systems aimed at streamlining diagnostics in urgent care and low-resource settings
- As global healthcare systems strive to improve patient outcomes, reduce diagnostic delays, and expand access to quality care, the continued evolution of point-of-care molecular diagnostics is poised to unlock new opportunities in both developed and emerging markets
Restraint/Challenge
“Increasing Regulatory Approvals”
- The growing number of regulatory approvals and compliance requirements presents a significant challenge for the molecular diagnostics market. While approvals are essential for ensuring safety and efficacy, the complexity and variability of global regulatory processes can slow product development and market entry
- This challenge is particularly pronounced for companies seeking to launch innovative diagnostic technologies across multiple regions, as they must navigate differing standards, documentation, and approval timelines.
- Lengthy approval processes can delay the availability of critical diagnostic tools, increase development costs, and create barriers for smaller or emerging players in the market
For instance,
- Startups often face extended timeframes when seeking FDA or CE mark approval for novel molecular assays, which can hinder commercialization strategies
- Without streamlined regulatory pathways and international harmonization, the molecular diagnostics market may face delays in innovation rollout, limiting timely access to advanced diagnostics and impacting patient care outcomes
Molecular Diagnostics Market Scope
The market is segmented on the basis of products, technology, application, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Products |
|
|
By Technology |
|
|
By Application |
|
|
By End User
|
|
Molecular Diagnostics Market Regional Analysis
“North America is the Dominant Region in the Molecular Diagnostics Market”
- North America dominates the molecular diagnostics market, driven by the increased healthcare expenditure, advanced healthcare facilities, and a well-established healthcare system that supports innovation and accessibility
- U.S. holds a significant share due to its high per capita healthcare spending, a large number of research institutions and biotech companies, and widespread consumer awareness about the importance of early disease detection and personalized medicine
- Leading companies in North America continue to innovate with next-generation diagnostic platforms, integrating artificial intelligence, molecular biology advancements, and cloud technologies. This has resulted in more efficient, scalable, and accurate diagnostic solutions that improve patient outcomes and healthcare delivery
- With strong infrastructure, an aging population, and rising consumer demand for precision healthcare, North America is expected to maintain its position as the largest and most advanced market for molecular diagnostics throughout the forecast period from 2025 to 2032
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is expected to witness the highest growth rate in the molecular diagnostics market, driven by rapid demographic shifts, an aging population, and an increasing prevalence of diseases such as cancer and infectious conditions
- Countries such as China, India, and Japan are leading the regional growth due to their expanding healthcare infrastructure, government initiatives to improve healthcare access, and rising awareness about early disease detection
- The region is also experiencing significant advancements in healthcare technology, including telemedicine, genomics, and mobile health diagnostics, which are boosting demand for advanced molecular diagnostic tools capable of handling large patient volumes and offering accurate, cost-effective testing
- With increasing healthcare digitization, government support for biotechnology advancements, and the growing adoption of personalized medicine, Asia-Pacific is positioned as the fastest-growing regional market for molecular diagnostics. As healthcare systems continue to evolve, Asia-Pacific will continue to expand its market share from 2025 to 2032, becoming a major hub for molecular diagnostic innovation and adoption
Molecular Diagnostics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd (Switzerland)
- Hologic, Inc. (U.S.)
- bioMérieux SA (France)
- Abbott Laboratories (U.S.)
- QIAGEN N.V. (Netherlands)
- Thermo Fisher Scientific Inc. (U.S.)
- Siemens AG (Germany)
- Danaher Corporation (U.S.)
- Myriad Genetics, Inc. (U.S.)
- Illumina, Inc. (U.S.)
- Agilent Technologies, Inc. (U.S.)
- Becton, Dickinson and Company (BD) (U.S.)
- DiaSorin S.p.A. (Italy)
- Grifols, S.A. (Spain)
- QuidelOrtho Corporation (U.S.)
- Genetic Signatures Limited (Australia)
- MDxHealth SA (Belgium)
- Exact Sciences Corporation (U.S.)
- Biocartis Group NV (Belgium)
- TBG Diagnostics Limited (Australia)
- GenMark Diagnostics, Inc. (U.S.)
- Luminex Corporation (U.S.)
- HTG Molecular Diagnostics, Inc. (U.S.)
- Vela Diagnostics (Singapore)
- Amoy Diagnostics Co., Ltd. (China)
- Molbio Diagnostics Pvt. Ltd. (India)
- geneOmbio Technologies Pvt. Ltd. (India)
Latest Developments in Global Molecular Diagnostics Market
- In 2021, Roche completed the acquisition of TIB Molbiol Group. TIB Molbiol Group has about 45 CE-IVD-approved assays for diagnosing inherited genetic testing, infectious diseases, transplant medicine, and hematology testing
- In 2020, Roche Diagnostics India launched the Cobas 8800 and the Cobas 6800 at the National Institute of Cholera and Enteric Diseases, Kolkata, to help the SARS CoV-2 diagnostic testing. The Roche Cobas 6800/8800 systems deliver test findings in a time of three and a half hours and allow greater operational efficiency, flexibility, and the quickest time-to-results
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL MOLECULAR DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL MOLECULAR DIAGNOSTICS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL MOLECULAR DIAGNOSTICS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER'S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNOLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 INSTALLED BASE DATA
15 VALUE CHAIN ANALYSIS
16 HEALTHCARE ECONOMY
16.1 HEALTHCARE EXPENDITURE
16.2 CAPITAL EXPENDITURE
16.3 CAPEX TRENDS
16.4 CAPEX ALLOCATION
16.5 FUNDING SOURCES
16.6 INDUSTRY BENCHMARKS
16.7 GDP RATION IN OVERALL GDP
16.8 HEALTHCARE SYSTEM STRUCTURE
16.9 GOVERNMENT POLICIES
16.1 ECONOMIC DEVELOPMENT
17 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY PRODUCT AND SERVICES
17.1 OVERVIEW
17.2 PRODUCT
17.2.1 INSTRUMENTS
17.2.1.1. BY TYPE
17.2.1.1.1. FULLY AUTOMATED
17.2.1.1.2. SEMI-AUTOMATED
17.2.1.1.3. MANUAL
17.2.1.2. BY MODALITY
17.2.1.2.1. PORTABLE
17.2.1.2.2. STANDALONE
17.2.1.2.3. BENCHTOP
17.2.2 REAGENTS AND KITS
17.2.2.1. KITS
17.2.2.1.1. DNA EXTRACTION AND PURIFICATION KITS
17.2.2.1.1.1 PLASMID MINIPREP AND MAXIPREP KITS
17.2.2.1.1.2 PLASMID PURIFICATION KITS
17.2.2.1.1.3 PCR PURIFICATION KITS
17.2.2.1.1.4 GENOMIC DNA PURIFICATION KITS
17.2.2.1.1.5 OTHERS
17.2.2.1.2. RNAI AND RNA REAGENTS
17.2.2.1.3. NUCLEIC ACID PURIFICATION KITS
17.2.2.1.4. NUCLEIC ACID SYNTHESIS KITS, BUFFERS & REAGENTS
17.2.2.1.5. HPV KITS
17.2.2.1.6. TB KITS
17.2.2.1.7. DEPLETION KITS
17.2.2.1.8. OTHERS
17.2.2.2. QC SETS AND PANELS
17.2.2.2.1. CONTROL PANEL
17.2.2.2.1.1 MULTIPLEX VAGINAL CONTROL PANEL
17.2.2.2.1.2 HUMAN PAPILLOMAVIRUS (HPV) CONTROL PANEL
17.2.2.2.1.3 CANDIDA VAGINITIS/TRICHOMONAS VAGINALIS (CV/TV) CONTROL PANEL
17.2.2.2.1.4 CT/NG CONTROL PANEL
17.2.2.2.1.5 BACTERIAL VAGINOSIS (BV) CONTROL PANEL
17.2.2.2.1.6 CELLULARITY CONTROL
17.2.2.2.1.7 RESPIRATORY CONTROL PANEL
17.2.2.2.1.8 FLU/RSV/SARS-COV-2 CONTROL PANEL
17.2.2.2.1.8.1. NXG CONTROL PANEL
17.2.2.2.1.8.2. COMPLETE CONTROL PANEL
17.2.2.2.1.8.3. OTHERS
17.2.2.2.1.9 MPN PANEL
17.2.2.2.1.10 AML PANEL
17.2.2.2.1.11 QC PANEL
17.2.2.2.1.12 ALL PANEL
17.2.2.2.1.13 VERIFICATION PANEL
17.2.2.2.1.14 OTHERS
17.2.2.3. MUTATIONS DETECTION KIT
17.2.2.3.1. KRAS PCR KIT
17.2.2.3.2. EGFR KIT
17.2.2.3.3. BRAF MUTATION KIT
17.2.2.3.4. AML1-ETO KIT
17.2.2.3.5. NRAS MUTATION KIT
17.2.2.3.6. CALR KIT
17.2.2.3.7. FLT3 MUTATION DETECTION
17.2.2.3.8. C-KIT MUTATION DETECTION KIT
17.2.2.3.9. MGMT METHYLATION DETECTION KIT
17.2.2.3.10. CBFB-MYH11 KIT
17.2.2.3.11. GENE FUSIONS DETECTION KIT
17.2.2.3.12. OTHER KITS AND ASSAYS
17.2.2.4. ENZYMES
17.2.2.4.1. POLYMERASES
17.2.2.4.2. LIGASES
17.2.2.4.3. RESTRICTION ENDONUCLEASES
17.2.2.4.4. REVERSE TRANSCRIPTASES
17.2.2.4.5. PHOSPHATASES
17.2.2.4.6. PROTEASES AND PROTEINASES
17.2.2.4.7. DNA LADDERS
17.2.2.4.8. OTHER ENZYMES
17.2.2.5. BUFFERS
17.2.2.6. PRIMERS
17.2.2.7. OTHERS
17.3 SOFTWARE AND SERVICES
17.3.1 SERVICES
17.3.1.1. INSTRUMENT REPAIR SERVICES
17.3.1.1.1. ON-SITE REPAIR SERVICES
17.3.1.1.2. OFF-SITE REPAIR SERVICES
17.3.1.2. TRAINING SERVICES
17.3.1.2.1. PCR BASED MOLECULAR DIAGNOSTICS
17.3.1.2.2. IMMUNOHISTOCHEMISTRY (IHC)
17.3.1.2.3. VIRAL LOAD TESTING
17.3.1.2.4. INTERPHASE CHROMOSOME PROFILING (ICP)
17.3.1.2.5. OTHERS
17.3.1.3. COMPLIANCE SERVICES
17.3.1.3.1. IQ/OQ & PM/OQ SERVICES
17.3.1.3.2. INSTALLATION SERVICES
17.3.1.3.3. VALIDATION SERVICES
17.3.1.3.4. OTHER SERVICES
17.3.1.4. SCALABLE AUTOMATION SERVICES
17.3.1.4.1. AUTOMATION FOR HIGH-CONTENT SCREENING (HCS)
17.3.1.4.2. AUTOMATION FOR HIGH-THROUGHPUT PLATE-BASED ASSAYS
17.3.1.4.3. AUTOMATION FOR HIGH-THROUGHPUT CLONE SCREENING
17.3.1.5. CALIBRATION SERVICES
17.3.1.6. MAINTENANCE SERVICES
17.3.1.7. INSTRUMENT RELOCATION SERVICES
17.3.1.7.1. PLATE RECERTIFICATION
17.3.1.7.2. FACTORY APPROVED PARTS
17.3.1.7.3. INSTRUMENT PERFORMANCE EVALUATION
17.3.1.7.4. OTHER SERVICES
17.3.1.8. HARDWARE CUSTOMIZATION
17.3.1.9. PERFORMANCE ASSURANCE SERVICES
17.3.1.10. DESIGN AND DEVELOPMENT SERVICES
17.3.1.11. SUPPLY CHAIN SOLUTIONS
17.3.1.12. CLINICAL RESEARCH SERVICES
17.3.1.13. OTHER SERVICES
17.3.2 SOFTWARE
17.3.2.1. BY TYPE
17.3.2.1.1. INTEGRATED
17.3.2.1.2. STANDALONE
17.3.2.2. BY DEPLOYMENT
17.3.2.2.1. CLOUD BASES
17.3.2.2.2. ON-PREMISES
17.3.2.2.3. HYBRID
18 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY TECHNOLOGY
18.1 OVERVIEW
18.2 POLYMERASE CHAIN REACTION (PCR)
18.2.1 BY TYPE
18.2.1.1. REAL-TIME PCR
18.2.1.2. DIGITAL PCR
18.2.1.3. REVERSE TRANSCRIPTASE PCR
18.2.1.4. QUANTITATIVE FLUORESCENT PCR
18.2.1.5. COLD PCR
18.2.1.6. OTHERS
18.2.2 BY PRODUCT AND SERVICES
18.2.2.1. PRODUCT
18.2.2.2. SERVICES AND SOFTWARE
18.3 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
18.3.1 BY PRODUCT AND SERVICES
18.3.1.1. PRODUCT
18.3.1.2. SERVICES AND SOFTWARE
18.4 NEXT GENERATION SEQUENCING (NGS)
18.4.1 BY PRODUCT AND SERVICES
18.4.1.1. PRODUCT
18.4.1.2. SERVICES AND SOFTWARE
18.5 CYTOGENETICS
18.5.1 BY PRODUCT AND SERVICES
18.5.1.1. PRODUCT
18.5.1.2. SERVICES AND SOFTWARE
18.6 CAPILLARY ELECTROPHORESIS
18.6.1 BY PRODUCT AND SERVICES
18.6.1.1. PRODUCT
18.6.1.2. SERVICES AND SOFTWARE
18.7 IN SITU HYBRIDIZATION (ISH OR FISH)
18.7.1 BY PRODUCT AND SERVICES
18.7.1.1. PRODUCT
18.7.1.2. SERVICES AND SOFTWARE
18.8 MOLECULAR IMAGING
18.8.1 BY TYPE
18.8.1.1. OPTICAL IMAGING
18.8.1.2. FDG-PET
18.8.1.3. OTHERS
18.8.2 BY PRODUCT AND SERVICES
18.8.2.1. PRODUCT
18.8.2.2. SERVICES AND SOFTWARE
18.9 MASS SPECTROMETRY (MS)
18.9.1 BY PRODUCT AND SERVICES
18.9.1.1. PRODUCT
18.9.1.2. SERVICES AND SOFTWARE
18.1 CHIPS AND MICROARRAY
18.10.1 BY PRODUCT AND SERVICES
18.10.1.1. PRODUCT
18.10.1.2. SERVICES AND SOFTWARE
18.11 OTHERS
19 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 ONCOLOGY TESTING
19.2.1 ONCOLOGY, BY CANCER TYPE
19.2.1.1. BREAST CANCER
19.2.1.1.1. MARKET VALUE (USD MILLION)
19.2.1.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.1.1.3. AVERAGE TEST COST (USD)
19.2.1.2. COLORECTAL CANCER
19.2.1.2.1. MARKET VALUE (USD MILLION)
19.2.1.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.1.2.3. AVERAGE TEST COST (USD)
19.2.1.3. LUNG CANCER
19.2.1.3.1. MARKET VALUE (USD MILLION)
19.2.1.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.1.3.3. AVERAGE TEST COST (USD)
19.2.1.4. PROSTATE CANCER
19.2.1.4.1. MARKET VALUE (USD MILLION)
19.2.1.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.1.4.3. AVERAGE TEST COST (USD)
19.2.1.5. OTHERS
19.2.2 ONCOLOGY, BY TECHNOLOGY
19.2.2.1. POLYMERASE CHAIN REACTION (PCR)
19.2.2.1.1. MARKET VALUE (USD MILLION)
19.2.2.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.1.3. AVERAGE TEST COST (USD)
19.2.2.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.2.2.2.1. MARKET VALUE (USD MILLION)
19.2.2.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.2.3. AVERAGE TEST COST (USD)
19.2.2.3. NEXT GENERATION SEQUENCING (NGS)
19.2.2.3.1. MARKET VALUE (USD MILLION)
19.2.2.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.3.3. AVERAGE TEST COST (USD)
19.2.2.4. CYTOGENETICS
19.2.2.4.1. MARKET VALUE (USD MILLION)
19.2.2.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.4.3. AVERAGE TEST COST (USD)
19.2.2.5. CAPILLARY ELECTROPHORESIS
19.2.2.5.1. MARKET VALUE (USD MILLION)
19.2.2.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.5.3. AVERAGE TEST COST (USD)
19.2.2.6. IN SITU HYBRIDIZATION (ISH OR FISH)
19.2.2.6.1. MARKET VALUE (USD MILLION)
19.2.2.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.6.3. AVERAGE TEST COST (USD)
19.2.2.7. MOLECULAR IMAGING
19.2.2.7.1. MARKET VALUE (USD MILLION)
19.2.2.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.7.3. AVERAGE TEST COST (USD)
19.2.2.8. MASS SPECTROMETRY (MS)
19.2.2.8.1. MARKET VALUE (USD MILLION)
19.2.2.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.8.3. AVERAGE TEST COST (USD)
19.2.2.9. CHIPS AND MICROARRAY
19.2.2.9.1. MARKET VALUE (USD MILLION)
19.2.2.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.2.2.9.3. AVERAGE TEST COST (USD)
19.2.2.10. OTHERS
19.3 PHARMACOGENOMICS
19.3.1 POLYMERASE CHAIN REACTION (PCR)
19.3.1.1. MARKET VALUE (USD MILLION)
19.3.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.1.3. AVERAGE TEST COST (USD)
19.3.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.3.2.1. MARKET VALUE (USD MILLION)
19.3.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.2.3. AVERAGE TEST COST (USD)
19.3.3 NEXT GENERATION SEQUENCING (NGS)
19.3.3.1. MARKET VALUE (USD MILLION)
19.3.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.3.3. AVERAGE TEST COST (USD)
19.3.4 CYTOGENETICS
19.3.4.1. MARKET VALUE (USD MILLION)
19.3.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.4.3. AVERAGE TEST COST (USD)
19.3.5 CAPILLARY ELECTROPHORESIS
19.3.5.1. MARKET VALUE (USD MILLION)
19.3.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.5.3. AVERAGE TEST COST (USD)
19.3.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.3.6.1. MARKET VALUE (USD MILLION)
19.3.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.6.3. AVERAGE TEST COST (USD)
19.3.7 MOLECULAR IMAGING
19.3.7.1. MARKET VALUE (USD MILLION)
19.3.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.7.3. AVERAGE TEST COST (USD)
19.3.8 MASS SPECTROMETRY (MS)
19.3.8.1. MARKET VALUE (USD MILLION)
19.3.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.8.3. AVERAGE TEST COST (USD)
19.3.9 CHIPS AND MICROARRAY
19.3.9.1. MARKET VALUE (USD MILLION)
19.3.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.3.9.3. AVERAGE TEST COST (USD)
19.3.10 OTHERS
19.4 MICROBIOLOGY
19.4.1 POLYMERASE CHAIN REACTION (PCR)
19.4.1.1. MARKET VALUE (USD MILLION)
19.4.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.1.3. AVERAGE TEST COST (USD)
19.4.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.4.2.1. MARKET VALUE (USD MILLION)
19.4.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.2.3. AVERAGE TEST COST (USD)
19.4.3 NEXT GENERATION SEQUENCING (NGS)
19.4.3.1. MARKET VALUE (USD MILLION)
19.4.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.3.3. AVERAGE TEST COST (USD)
19.4.4 CYTOGENETICS
19.4.4.1. MARKET VALUE (USD MILLION)
19.4.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.4.3. AVERAGE TEST COST (USD)
19.4.5 CAPILLARY ELECTROPHORESIS
19.4.5.1. MARKET VALUE (USD MILLION)
19.4.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.5.3. AVERAGE TEST COST (USD)
19.4.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.4.6.1. MARKET VALUE (USD MILLION)
19.4.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.6.3. AVERAGE TEST COST (USD)
19.4.7 MOLECULAR IMAGING
19.4.7.1. MARKET VALUE (USD MILLION)
19.4.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.7.3. AVERAGE TEST COST (USD)
19.4.8 MASS SPECTROMETRY (MS)
19.4.8.1. MARKET VALUE (USD MILLION)
19.4.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.8.3. AVERAGE TEST COST (USD)
19.4.9 CHIPS AND MICROARRAY
19.4.9.1. MARKET VALUE (USD MILLION)
19.4.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.4.9.3. AVERAGE TEST COST (USD)
19.4.10 OTHERS
19.5 PRENATAL TESTS
19.5.1 SICKLE CELL DISEASE
19.5.1.1. MARKET VALUE (USD MILLION)
19.5.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.5.1.3. AVERAGE TEST COST (USD)
19.5.2 CYSTIC FIBROSIS
19.5.2.1. MARKET VALUE (USD MILLION)
19.5.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.5.2.3. AVERAGE TEST COST (USD)
19.5.3 TAY-SACHS DISEASE
19.5.3.1. MARKET VALUE (USD MILLION)
19.5.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.5.3.3. AVERAGE TEST COST (USD)
19.5.4 OTHERS
19.6 TISSUE TYPING TEST
19.6.1 POLYMERASE CHAIN REACTION (PCR)
19.6.1.1. MARKET VALUE (USD MILLION)
19.6.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.1.3. AVERAGE TEST COST (USD)
19.6.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.6.2.1. MARKET VALUE (USD MILLION)
19.6.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.2.3. AVERAGE TEST COST (USD)
19.6.3 NEXT GENERATION SEQUENCING (NGS)
19.6.3.1. MARKET VALUE (USD MILLION)
19.6.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.3.3. AVERAGE TEST COST (USD)
19.6.4 CYTOGENETICS
19.6.4.1. MARKET VALUE (USD MILLION)
19.6.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.4.3. AVERAGE TEST COST (USD)
19.6.5 CAPILLARY ELECTROPHORESIS
19.6.5.1. MARKET VALUE (USD MILLION)
19.6.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.5.3. AVERAGE TEST COST (USD)
19.6.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.6.6.1. MARKET VALUE (USD MILLION)
19.6.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.6.3. AVERAGE TEST COST (USD)
19.6.7 MOLECULAR IMAGING
19.6.7.1. MARKET VALUE (USD MILLION)
19.6.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.7.3. AVERAGE TEST COST (USD)
19.6.8 MASS SPECTROMETRY (MS)
19.6.8.1. MARKET VALUE (USD MILLION)
19.6.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.8.3. AVERAGE TEST COST (USD)
19.6.9 CHIPS AND MICROARRAY
19.6.9.1. MARKET VALUE (USD MILLION)
19.6.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.6.9.3. AVERAGE TEST COST (USD)
19.6.10 OTHERS
19.7 BLOOD SCREENING
19.7.1 POLYMERASE CHAIN REACTION (PCR)
19.7.1.1. MARKET VALUE (USD MILLION)
19.7.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.1.3. AVERAGE TEST COST (USD)
19.7.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.7.2.1. MARKET VALUE (USD MILLION)
19.7.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.2.3. AVERAGE TEST COST (USD)
19.7.3 NEXT GENERATION SEQUENCING (NGS)
19.7.3.1. MARKET VALUE (USD MILLION)
19.7.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.3.3. AVERAGE TEST COST (USD)
19.7.4 CYTOGENETICS
19.7.4.1. MARKET VALUE (USD MILLION)
19.7.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.4.3. AVERAGE TEST COST (USD)
19.7.5 CAPILLARY ELECTROPHORESIS
19.7.5.1. MARKET VALUE (USD MILLION)
19.7.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.5.3. AVERAGE TEST COST (USD)
19.7.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.7.6.1. MARKET VALUE (USD MILLION)
19.7.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.6.3. AVERAGE TEST COST (USD)
19.7.7 MOLECULAR IMAGING
19.7.7.1. MARKET VALUE (USD MILLION)
19.7.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.7.3. AVERAGE TEST COST (USD)
19.7.8 MASS SPECTROMETRY (MS)
19.7.8.1. MARKET VALUE (USD MILLION)
19.7.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.8.3. AVERAGE TEST COST (USD)
19.7.9 CHIPS AND MICROARRAY
19.7.9.1. MARKET VALUE (USD MILLION)
19.7.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.7.9.3. AVERAGE TEST COST (USD)
19.7.10 OTHERS
19.8 CARDIOVASCULAR DISEASES TESTING
19.8.1 POLYMERASE CHAIN REACTION (PCR)
19.8.1.1. MARKET VALUE (USD MILLION)
19.8.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.1.3. AVERAGE TEST COST (USD)
19.8.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.8.2.1. MARKET VALUE (USD MILLION)
19.8.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.2.3. AVERAGE TEST COST (USD)
19.8.3 NEXT GENERATION SEQUENCING (NGS)
19.8.3.1. MARKET VALUE (USD MILLION)
19.8.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.3.3. AVERAGE TEST COST (USD)
19.8.4 CYTOGENETICS
19.8.4.1. MARKET VALUE (USD MILLION)
19.8.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.4.3. AVERAGE TEST COST (USD)
19.8.5 CAPILLARY ELECTROPHORESIS
19.8.5.1. MARKET VALUE (USD MILLION)
19.8.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.5.3. AVERAGE TEST COST (USD)
19.8.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.8.6.1. MARKET VALUE (USD MILLION)
19.8.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.6.3. AVERAGE TEST COST (USD)
19.8.7 MOLECULAR IMAGING
19.8.7.1. MARKET VALUE (USD MILLION)
19.8.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.7.3. AVERAGE TEST COST (USD)
19.8.8 MASS SPECTROMETRY (MS)
19.8.8.1. MARKET VALUE (USD MILLION)
19.8.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.8.3. AVERAGE TEST COST (USD)
19.8.9 CHIPS AND MICROARRAY
19.8.9.1. MARKET VALUE (USD MILLION)
19.8.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.8.9.3. AVERAGE TEST COST (USD)
19.8.10 OTHERS
19.9 NEUROLOGICAL DISEASES TESTING
19.9.1 POLYMERASE CHAIN REACTION (PCR)
19.9.1.1. MARKET VALUE (USD MILLION)
19.9.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.1.3. AVERAGE TEST COST (USD)
19.9.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.9.2.1. MARKET VALUE (USD MILLION)
19.9.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.2.3. AVERAGE TEST COST (USD)
19.9.3 NEXT GENERATION SEQUENCING (NGS)
19.9.3.1. MARKET VALUE (USD MILLION)
19.9.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.3.3. AVERAGE TEST COST (USD)
19.9.4 CYTOGENETICS
19.9.4.1. MARKET VALUE (USD MILLION)
19.9.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.4.3. AVERAGE TEST COST (USD)
19.9.5 CAPILLARY ELECTROPHORESIS
19.9.5.1. MARKET VALUE (USD MILLION)
19.9.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.5.3. AVERAGE TEST COST (USD)
19.9.6 IN SITU HYBRIDIZATION (ISH OR FISH)
19.9.6.1. MARKET VALUE (USD MILLION)
19.9.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.6.3. AVERAGE TEST COST (USD)
19.9.7 MOLECULAR IMAGING
19.9.7.1. MARKET VALUE (USD MILLION)
19.9.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.7.3. AVERAGE TEST COST (USD)
19.9.8 MASS SPECTROMETRY (MS)
19.9.8.1. MARKET VALUE (USD MILLION)
19.9.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.8.3. AVERAGE TEST COST (USD)
19.9.9 CHIPS AND MICROARRAY
19.9.9.1. MARKET VALUE (USD MILLION)
19.9.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.9.9.3. AVERAGE TEST COST (USD)
19.9.10 OTHERS
19.1 INFECTIOUS DISEASES TESTING
19.10.1 BY TYPE
19.10.1.1. COVID-19
19.10.1.1.1. MARKET VALUE (USD MILLION)
19.10.1.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.1.3. AVERAGE TEST COST (USD)
19.10.1.2. HEPATITIS
19.10.1.2.1. MARKET VALUE (USD MILLION)
19.10.1.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.2.3. AVERAGE TEST COST (USD)
19.10.1.3. HIV
19.10.1.3.1. MARKET VALUE (USD MILLION)
19.10.1.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.3.3. AVERAGE TEST COST (USD)
19.10.1.4. CT/NG
19.10.1.4.1. MARKET VALUE (USD MILLION)
19.10.1.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.4.3. AVERAGE TEST COST (USD)
19.10.1.5. HAI
19.10.1.5.1. MARKET VALUE (USD MILLION)
19.10.1.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.5.3. AVERAGE TEST COST (USD)
19.10.1.6. HPV
19.10.1.6.1. MARKET VALUE (USD MILLION)
19.10.1.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.6.3. AVERAGE TEST COST (USD)
19.10.1.7. TUBERCULOSIS
19.10.1.7.1. MARKET VALUE (USD MILLION)
19.10.1.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.7.3. AVERAGE TEST COST (USD)
19.10.1.8. INFLUENZA
19.10.1.8.1. MARKET VALUE (USD MILLION)
19.10.1.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.1.8.3. AVERAGE TEST COST (USD)
19.10.1.9. VECTOR-BORNE DISEASE
19.10.1.9.1. MALERIA
19.10.1.9.1.1 MARKET VALUE (USD MILLION)
19.10.1.9.1.2 MARKET VOLUME (NUMBER OF TESTS)
19.10.1.9.1.3 AVERAGE TEST COST (USD)
19.10.1.9.2. DENGUE
19.10.1.9.2.1 MARKET VALUE (USD MILLION)
19.10.1.9.2.2 MARKET VOLUME (NUMBER OF TESTS)
19.10.1.9.2.3 AVERAGE TEST COST (USD)
19.10.1.9.3. ZIKA VIRUS
19.10.1.9.3.1 MARKET VALUE (USD MILLION)
19.10.1.9.3.2 MARKET VOLUME (NUMBER OF TESTS)
19.10.1.9.3.3 AVERAGE TEST COST (USD)
19.10.1.9.4. OTHERS
19.10.2 BY TECHNOLOGY
19.10.2.1. POLYMERASE CHAIN REACTION (PCR)
19.10.2.1.1. MARKET VALUE (USD MILLION)
19.10.2.1.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.1.3. AVERAGE TEST COST (USD)
19.10.2.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
19.10.2.2.1. MARKET VALUE (USD MILLION)
19.10.2.2.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.2.3. AVERAGE TEST COST (USD)
19.10.2.3. NEXT GENERATION SEQUENCING (NGS)
19.10.2.3.1. MARKET VALUE (USD MILLION)
19.10.2.3.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.3.3. AVERAGE TEST COST (USD)
19.10.2.4. CYTOGENETICS
19.10.2.4.1. MARKET VALUE (USD MILLION)
19.10.2.4.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.4.3. AVERAGE TEST COST (USD)
19.10.2.5. CAPILLARY ELECTROPHORESIS
19.10.2.5.1. MARKET VALUE (USD MILLION)
19.10.2.5.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.5.3. AVERAGE TEST COST (USD)
19.10.2.6. IN SITU HYBRIDIZATION (ISH OR FISH)
19.10.2.6.1. MARKET VALUE (USD MILLION)
19.10.2.6.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.6.3. AVERAGE TEST COST (USD)
19.10.2.7. MOLECULAR IMAGING
19.10.2.7.1. MARKET VALUE (USD MILLION)
19.10.2.7.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.7.3. AVERAGE TEST COST (USD)
19.10.2.8. MASS SPECTROMETRY (MS)
19.10.2.8.1. MARKET VALUE (USD MILLION)
19.10.2.8.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.8.3. AVERAGE TEST COST (USD)
19.10.2.9. CHIPS AND MICROARRAY
19.10.2.9.1. MARKET VALUE (USD MILLION)
19.10.2.9.2. MARKET VOLUME (NUMBER OF TESTS)
19.10.2.9.3. AVERAGE TEST COST (USD)
19.10.2.10. OTHERS
19.11 OTHERS
20 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY TESTING SITE
20.1 OVERVIEW
20.2 LABORATORY BASED
20.3 POINT OF CARE TESTING
21 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS CORE LABORATORIES
21.3 HOSPITAL ALTERNATIVE SITES
21.3.1 CLINICS
21.3.2 REFERENCE LABORATORIES
21.3.3 DIAGNOSTIC CENTERS
21.3.4 ACADEMIC & RESEARCH INSTITUTES
21.3.5 OTHERS
21.4 DECENTRALIZED TEST SITES
21.4.1 DECENTRALIZED CLINICAL LABORATORIES
21.4.2 PHARMACIES
21.4.3 HOMECARE SETTING
21.5 OTHERS
22 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDERS
22.3 RETAIL SALES
22.3.1 ONLINE SALES
22.3.2 OFFLINES SALES
22.4 OTHERS
23 GLOBAL MOLECULAR DIAGNOSTICS MARKET, BY COUNTRY
GLOBAL MOLECULAR DIAGNOSTICS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
23.1 NORTH AMERICA
23.1.1 U.S.
23.1.2 CANADA
23.1.3 MEXICO
23.2 EUROPE
23.2.1 GERMANY
23.2.2 FRANCE
23.2.3 U.K.
23.2.4 ITALY
23.2.5 SPAIN
23.2.6 RUSSIA
23.2.7 TURKEY
23.2.8 BELGIUM
23.2.9 NETHERLANDS
23.2.10 SWITZERLAND
23.2.11 REST OF EUROPE
23.3 ASIA-PACIFIC
23.3.1 JAPAN
23.3.2 CHINA
23.3.3 SOUTH KOREA
23.3.4 INDIA
23.3.5 AUSTRALIA
23.3.6 SINGAPORE
23.3.7 THAILAND
23.3.8 MALAYSIA
23.3.9 INDONESIA
23.3.10 PHILIPPINES
23.3.11 REST OF ASIA-PACIFIC
23.4 SOUTH AMERICA
23.4.1 BRAZIL
23.4.2 ARGENTINA
23.4.3 PERU
23.4.4 CHILE
23.4.5 COLOMBIA
23.4.6 VENEZUELA
23.4.7 REST OF SOUTH AMERICA
23.5 MIDDLE EAST AND AFRICA
23.5.1 SOUTH AFRICA
23.5.2 SAUDI ARABIA
23.5.3 UAE
23.5.4 EGYPT
23.5.5 ISRAEL
23.5.6 REST OF MIDDLE EAST AND AFRICA
23.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
24 GLOBAL SWINE AND POULTRY RESPIRATORY DISEASES TREATMENT MARKET , COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
24.5 MERGERS & ACQUISITIONS
24.6 NEW PRODUCT DEVELOPMENT & APPROVALS
24.7 EXPANSIONS
24.8 REGULATORY CHANGES
24.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 GLOBAL MOLECULAR DIAGNOSTICS MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
25.2 MERGERS & ACQUISITIONS
25.3 NEW PRODUCT DEVELOPMENT & APPROVALS
25.4 EXPANSIONS
25.5 REGULATORY CHANGES
25.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26 GLOBAL MOLECULAR DIAGNOSTICS MARKET, SWOT AND DBMR ANALYSIS
27 GLOBAL MOLECULAR DIAGNOSTICS MARKET, COMPANY PROFILE
27.1 ABBOTT
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 SIEMENS HEALTHCARE PRIVATE LIMITED
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 THERMO FISHER SCIENTIFIC INC.
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 BD
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 BIOMÉRIEUX SA
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 DANAHER CORPORATION
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 HOLOGIC, INC.
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 MYRIAD GENETICS, INC.
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 QIAGEN
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.1 AGILENT TECHNOLOGIES, INC.
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 QUIDEL CORPORATION.
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 BIO-RAD LABORATORIES, INC.
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 ILLUMINA, INC.
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 IMMUCOR (WERFEN, S.A.)
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 DIASORIN S.P.A
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 SD BIOSENSOR
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
27.17 F. HOFFMANN-LA ROCHE LTD
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPMENTS
27.18 GENEPATH DIAGNOSTICS
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPMENTS
27.19 EXACT SCIENCES CORPORATION
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPMENTS
27.2 GUARDANT HEALTH
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPMENTS
27.21 REVVITY INC.
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.21.5 RECENT DEVELOPMENTS
27.22 CARIS LIFE SCIENCES.
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.22.5 RECENT DEVELOPMENTS
27.23 RANDOX LABORATORIES LTD.
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.23.5 RECENT DEVELOPMENTS
27.24 DAAN GENE CO., LTD.
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.24.5 RECENT DEVELOPMENTS
27.25 JIANGSU MACRO & MICRO-TEST MED-TECH CO., LTD.
27.25.1 COMPANY OVERVIEW
27.25.2 REVENUE ANALYSIS
27.25.3 GEOGRAPHIC PRESENCE
27.25.4 PRODUCT PORTFOLIO
27.25.5 RECENT DEVELOPMENTS
27.26 GENOBIO PHARMACEUTICAL CO., LTD. (ERA BIOLOGY GROUP)
27.26.1 COMPANY OVERVIEW
27.26.2 REVENUE ANALYSIS
27.26.3 GEOGRAPHIC PRESENCE
27.26.4 PRODUCT PORTFOLIO
27.26.5 RECENT DEVELOPMENTS
27.27 LEPU MEDICAL TECHNOLOGY(BEIJING)CO.,LTD.
27.27.1 COMPANY OVERVIEW
27.27.2 REVENUE ANALYSIS
27.27.3 GEOGRAPHIC PRESENCE
27.27.4 PRODUCT PORTFOLIO
27.27.5 RECENT DEVELOPMENTS
27.28 PROMEGA CORPORATION
27.28.1 COMPANY OVERVIEW
27.28.2 REVENUE ANALYSIS
27.28.3 GEOGRAPHIC PRESENCE
27.28.4 PRODUCT PORTFOLIO
27.28.5 RECENT DEVELOPMENTS
27.29 TRIPLEX INTERNATIONAL BIOSCIENCES CO. LTD.
27.29.1 COMPANY OVERVIEW
27.29.2 REVENUE ANALYSIS
27.29.3 GEOGRAPHIC PRESENCE
27.29.4 PRODUCT PORTFOLIO
27.29.5 RECENT DEVELOPMENTS
27.3 MGMED
27.30.1 COMPANY OVERVIEW
27.30.2 REVENUE ANALYSIS
27.30.3 GEOGRAPHIC PRESENCE
27.30.4 PRODUCT PORTFOLIO
27.30.5 RECENT DEVELOPMENTS
27.31 MOLBIO DIAGNOSTICS PVT. LTD.
27.31.1 COMPANY OVERVIEW
27.31.2 REVENUE ANALYSIS
27.31.3 GEOGRAPHIC PRESENCE
27.31.4 PRODUCT PORTFOLIO
27.31.5 RECENT DEVELOPMENTS
27.32 GENOME DIAGNOSTICS PVT. LTD.
27.32.1 COMPANY OVERVIEW
27.32.2 REVENUE ANALYSIS
27.32.3 GEOGRAPHIC PRESENCE
27.32.4 PRODUCT PORTFOLIO
27.32.5 RECENT DEVELOPMENTS
27.33 VELA DIAGNOSTICS
27.33.1 COMPANY OVERVIEW
27.33.2 REVENUE ANALYSIS
27.33.3 GEOGRAPHIC PRESENCE
27.33.4 PRODUCT PORTFOLIO
27.33.5 RECENT DEVELOPMENTS
27.34 SHANGHAI CHUANGKUN BIO
27.34.1 COMPANY OVERVIEW
27.34.2 REVENUE ANALYSIS
27.34.3 GEOGRAPHIC PRESENCE
27.34.4 PRODUCT PORTFOLIO
27.34.5 RECENT DEVELOPMENTS
27.35 TRANSGEN BIOTECH CO., LTD.
27.35.1 COMPANY OVERVIEW
27.35.2 REVENUE ANALYSIS
27.35.3 GEOGRAPHIC PRESENCE
27.35.4 PRODUCT PORTFOLIO
27.35.5 RECENT DEVELOPMENTS
27.36 NANJING VAZYME BIOTECH CO.,LTD.
27.36.1 COMPANY OVERVIEW
27.36.2 REVENUE ANALYSIS
27.36.3 GEOGRAPHIC PRESENCE
27.36.4 PRODUCT PORTFOLIO
27.36.5 RECENT DEVELOPMENTS
27.37 WUXI NEST BIOTECHNOLOGY CO.,LTD
27.37.1 COMPANY OVERVIEW
27.37.2 REVENUE ANALYSIS
27.37.3 GEOGRAPHIC PRESENCE
27.37.4 PRODUCT PORTFOLIO
27.37.5 RECENT DEVELOPMENTS
27.38 HANGZHOU BIGFISH BIO-TECH CO., LTD.
27.38.1 COMPANY OVERVIEW
27.38.2 REVENUE ANALYSIS
27.38.3 GEOGRAPHIC PRESENCE
27.38.4 PRODUCT PORTFOLIO
27.38.5 RECENT DEVELOPMENTS
27.39 MAGGENOME TECHNOLOGIES PVT. LTD.
27.39.1 COMPANY OVERVIEW
27.39.2 REVENUE ANALYSIS
27.39.3 GEOGRAPHIC PRESENCE
27.39.4 PRODUCT PORTFOLIO
27.39.5 RECENT DEVELOPMENTS
27.4 YANENG BIOSCIENCE (SHENZHEN) CO., LTD.
27.40.1 COMPANY OVERVIEW
27.40.2 REVENUE ANALYSIS
27.40.3 GEOGRAPHIC PRESENCE
27.40.4 PRODUCT PORTFOLIO
27.40.5 RECENT DEVELOPMENTS
27.41 MERIDIAN BIOSCIENCE
27.41.1 COMPANY OVERVIEW
27.41.2 REVENUE ANALYSIS
27.41.3 GEOGRAPHIC PRESENCE
27.41.4 PRODUCT PORTFOLIO
27.41.5 RECENT DEVELOPMENTS
28 RELATED REPORTS
29 CONCLUSION
30 QUESTIONNAIRE
31 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.