Global Mrna Flu Vaccine Market
Market Size in USD Billion
CAGR :
% 
 USD
9.43 Billion
USD
19.20 Billion
2024
2032
USD
9.43 Billion
USD
19.20 Billion
2024
2032
| 2025 –2032 | |
| USD 9.43 Billion | |
| USD 19.20 Billion | |
|
|
|
|
MRNA Flu Vaccine Market Size
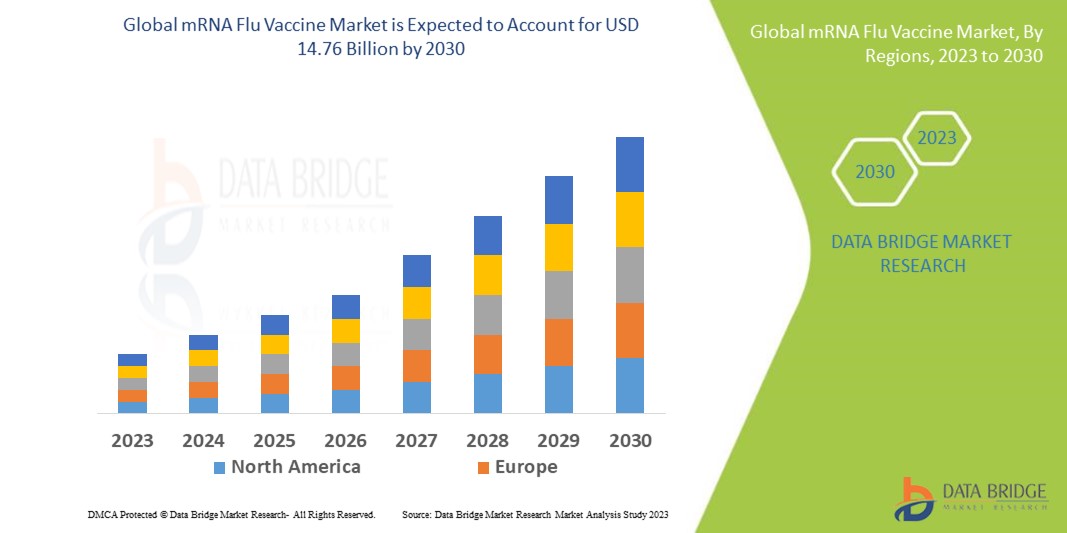
The global mRNA flu vaccine market size was valued at USD 9.43 billion in 2024 and is projected to reach USD 19.20 billion by 2032, with a CAGR of 9.30% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
MRNA Flu Vaccine Market Analysis
The mRNA flu vaccine market is rapidly evolving as mRNA technology reshapes the landscape of vaccine development. These vaccines utilize mRNA to instruct cells to produce antigens, triggering an immune response. With the success of mRNA-based COVID-19 vaccines, there is increasing interest in applying this technology to combat seasonal influenza. Key players, including Moderna and BioNTech, are advancing mRNA flu vaccine candidates, emphasizing improved efficacy, faster production, and adaptability to emerging strains. Recent developments include promising trial results and collaborations between biotech firms and governments to enhance mRNA vaccine capabilities. The market is expected to grow significantly, driven by rising awareness of influenza prevention, advancements in mRNA research, and the global focus on pandemic preparedness. However, challenges such as high costs and cold-chain logistics remain. Overall, the mRNA flu vaccine market holds immense potential to revolutionize flu prevention through innovative and effective solutions.
MRNA Flu Vaccine Market Trends
“Rapid Vaccine Development and Improvements”
The mRNA flu vaccine market is gaining traction as mRNA technology demonstrates its potential to revolutionize influenza prevention. These vaccines offer enhanced efficacy, faster production, and adaptability to emerging flu strains. Innovations in this market include platform technologies enabling rapid vaccine development and improvements in stability for better storage and distribution. A key trend is the integration of personalized vaccines, tailored to target specific populations or high-risk groups, enhancing overall immunization outcomes. Major players, such as Moderna and BioNTech, are actively investing in clinical trials and collaborations to advance mRNA flu vaccines. With the growing focus on pandemic preparedness and advancements in biotechnology, the mRNA flu vaccine market is set to transform traditional influenza vaccine approaches.
Report Scope and MRNA Flu Vaccine Market Segmentation
Attributes |
MRNA Flu Vaccine Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Key Market Players |
Moderna, Inc. (U.S.), BioNTech SE (Germany), CureVac SE (Germany), Novavax (U.S.), Sanofi (U.S.), INOVIO Pharmaceuticals (U.S.), Arcturus Therapeutics, Inc. (U.S.), etherna (Belgium), Valneva SE (France), Vaxart (U.S.), Entos Pharmaceuticals (Canada), Abnova Corporation (Taiwan), Anima Biotech Inc. (U.S.), Sarepta Therapeutics, Inc. (U.S.), IBIO, INC. (U.S.), Barinthus Biotherapeutics (U.K.), Alnylam Pharmaceuticals, Inc. (U.S.), Beam Therapeutics (U.S.), Chardan Healthcare Acquisition 2 Corp (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
MRNA Flu Vaccine Market Definition
An mRNA flu vaccine is a type of influenza vaccine that uses messenger RNA (mRNA) technology to instruct cells in the body to produce a protein similar to the virus's spike protein. This prompts the immune system to recognize and fight the actual virus if encountered later. Unlike traditional flu vaccines, which use inactivated or weakened virus strains, mRNA vaccines do not require the live virus to be grown in labs, allowing for faster production and adaptability to changing flu strains. The technology offers potential advantages in terms of speed, efficacy, and the ability to rapidly respond to emerging flu variants.
MRNA Flu Vaccine Market Dynamics
Drivers
- Rising Influenza Awareness
Growing awareness of the importance of flu vaccination, particularly during the flu season, is playing a significant role in driving the demand for more effective vaccines. As seasonal influenza can cause significant health risks, especially among vulnerable populations such as the elderly, children, and those with chronic health conditions, public health campaigns are emphasizing the need for timely vaccination. This increasing awareness is pushing individuals to seek out vaccines that offer improved efficacy and quicker protection. Consequently, there is a rising demand for advanced vaccine technologies such as mRNA, which offer better adaptability to flu strains and quicker production, driving the growth of the mRNA flu vaccine market.
- Increased Focus on Pandemic Preparedness
Governments and health organizations are prioritizing investments in innovative vaccine technologies to ensure a rapid and effective response to future influenza outbreaks and potential pandemics. The COVID-19 pandemic highlighted the vulnerabilities in global health systems and the importance of preparedness for emerging infectious diseases. In response, authorities are increasing funding for research and development in advanced vaccine technologies, such as mRNA, which offer faster production and adaptability to evolving virus strains. These investments aim to create robust vaccine infrastructures and ensure that the global community is better equipped to handle future flu outbreaks, driving the growth of the mRNA flu vaccine market.
Opportunities
- Enhanced Storage and Distribution Solutions
Advances in stabilizing mRNA vaccines for easier distribution and storage represent a significant market opportunity for widespread global vaccination. One of the main challenges for mRNA vaccines is their requirement for ultra-cold storage, which can complicate distribution, especially in regions with limited infrastructure. However, recent breakthroughs in stabilizing mRNA formulations are making it possible to store vaccines at higher temperatures, improving accessibility and reducing logistical challenges. This progress enables vaccines to reach underserved and remote areas more effectively, broadening the market for mRNA flu vaccines. As storage and distribution solutions improve, the opportunity for widespread adoption and global vaccination campaigns expands, driving growth in the mRNA flu vaccine market.
- Development of Personalized Flu Vaccines
The development of personalized flu vaccines tailored to specific populations or individuals presents a significant market opportunity by enhancing vaccine effectiveness. Personalization allows for vaccines that are specifically designed to address the unique needs of different age groups, genetic profiles, or individuals with underlying health conditions. For instance, flu vaccines customized for the elderly or immunocompromised individuals could offer improved protection and reduce the risk of severe illness. This approach could also address varying flu strains more precisely, increasing overall vaccination efficacy. As personalized medicine gains traction, this strategy could create new market segments, driving demand for mRNA-based flu vaccines and further expanding market growth.
Restraints/Challenges
- Competition from Traditional Flu Vaccines
Traditional flu vaccines, which have been used for decades, are well-established and dominate the global market. These vaccines have a large, loyal user base and extensive distribution networks, making them the go-to option for seasonal flu prevention. As a result, the adoption of mRNA flu vaccines faces resistance due to the strong market presence of conventional vaccines. Many healthcare systems and consumers may be hesitant to switch to a new technology, especially when traditional vaccines have a proven track record. This market inertia and the entrenched preference for traditional vaccines present a significant challenge for the widespread adoption of mRNA flu vaccines.
- High Production Costs
The production of mRNA vaccines is significantly more expensive than traditional flu vaccines, which could be a major restraint to their widespread adoption, especially in lower-income regions. The complex manufacturing processes and the need for specialized equipment and materials contribute to the higher cost of mRNA vaccines. In contrast, traditional flu vaccines have lower production costs due to established manufacturing techniques and economies of scale. This cost disparity could limit access to mRNA vaccines in economically disadvantaged areas, where affordability is a critical factor. Consequently, the higher cost of mRNA flu vaccines may slow their global distribution and adoption, particularly in underserved markets.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
MRNA Flu Vaccine Market Scope
The market is segmented on the basis of types, product type, route of administration, end users, and distribution channel. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Non-replicating mRNA
- In Vivo Self-Replicating mRNA
- In Vitro Dendritic Cell Non-Replicating mRNA Vaccine
Product Type
- mRNA-1010
- mRNA-1020
- mRNA-1030
Route of Administration
- Intravenous
- Intramuscular
- Others
End-Users
- Clinic
- Hospital
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
MRNA Flu Vaccine Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, types, product type, route of administration, end users, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the global mRNA flu vaccine market, driven by the presence of prominent industry players and substantial investments in research and development. The region benefits from high healthcare spending and a robust healthcare infrastructure, which supports the adoption of advanced vaccine technologies. These factors contribute to North America's dominance in the growing mRNA vaccine sector.
The Asia-Pacific region is projected to experience rapid and significant growth in the global mRNA flu vaccine market from 2025 to 2032. This growth is fueled by increasing research and development initiatives, rising investments in the healthcare sector, and strong government support for innovative vaccine technologies. These factors collectively create a promising environment for the expansion of mRNA flu vaccines in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
MRNA Flu Vaccine Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
MRNA Flu Vaccine Market Leaders Operating in the Market Are:
- Moderna, Inc. (U.S.)
- BioNTech SE (Germany)
- CureVac SE (Germany)
- Novavax (U.S.)
- Sanofi (U.S.)
- INOVIO Pharmaceuticals (U.S.)
- Arcturus Therapeutics, Inc. (U.S.)
- etherna (Belgium)
- Valneva SE (France)
- Vaxart (U.S.)
- Entos Pharmaceuticals (Canada)
- Abnova Corporation (Taiwan)
- Anima Biotech Inc. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- IBIO, INC. (U.S.)
- Barinthus Biotherapeutics (U.K.)
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Beam Therapeutics (U.S.)
- Chardan Healthcare Acquisition 2 Corp (U.S.)
Latest Developments in MRNA Flu Vaccine Market
- In October 2023, Moderna Inc. revealed the results of its mRNA-based vaccine targeting both influenza and COVID-19, showcasing its potential for combined respiratory disease prevention
- In May 2023, GSK, in partnership with CureVac, launched a Phase I/II clinical trial for their mRNA-based influenza vaccine, marking a significant step in advancing innovative vaccine solutions
- In September 2022, Pfizer Inc. began a Phase 3 clinical trial for its mRNA-based influenza vaccine, enrolling 25,000 U.S. adults to assess safety, efficacy, immunogenicity, and tolerability of its quadrivalent modified RNA flu vaccine
- In March 2022, Seqirus received Health Canada approval for Flucelvax quadrivalent, a cell-based influenza vaccine for individuals aged two and older, extending eligibility to children aged 2 to 18 years
- In September 2021, Sanofi acquired Translate Bio, a clinical-stage mRNA therapeutics company, to accelerate the development of novel vaccines for influenza and other seasonal diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.