Global Myasthenia Gravis Disease Market
Market Size in USD Million
CAGR :
% 
 USD
861.02 Million
USD
1,969.99 Million
2024
2032
USD
861.02 Million
USD
1,969.99 Million
2024
2032
| 2025 –2032 | |
| USD 861.02 Million | |
| USD 1,969.99 Million | |
|
|
|
|
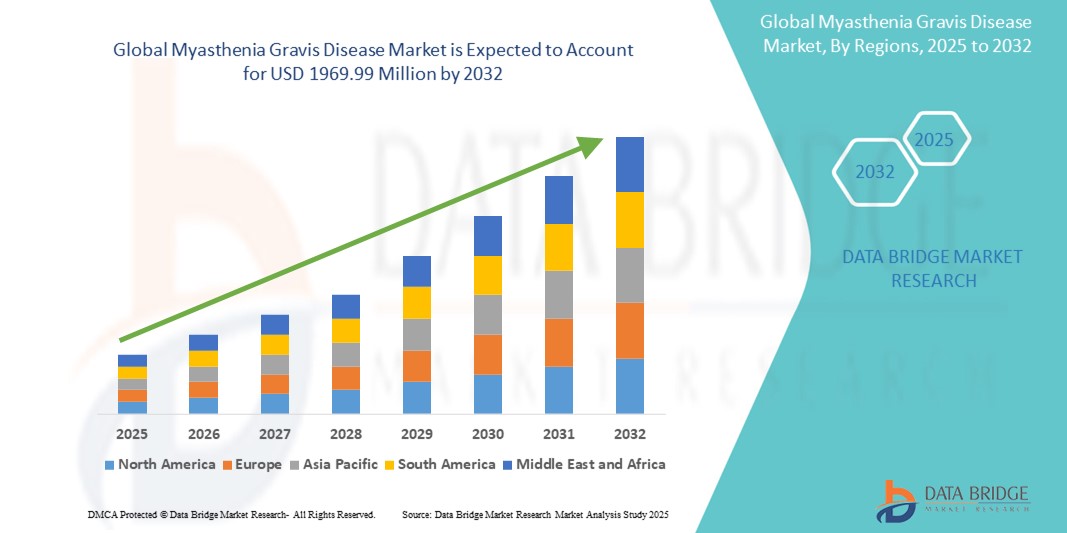
Myasthenia Gravis Disease Market Size
- The global myasthenia gravis disease market size was valued at USD 861.02 million in 2024 and is expected to reach USD 1969.99 million by 2032, at a CAGR of 10.9% during the forecast period
- The market growth is primarily driven by increasing awareness of myasthenia gravis, advancements in diagnostic techniques, and the development of novel treatment options, including targeted therapies and biologics
- Rising prevalence of autoimmune diseases, coupled with growing healthcare expenditure and demand for personalized medicine, is further propelling the adoption of advanced treatment solutions for myasthenia gravis
Myasthenia Gravis Disease Market Analysis
- Myasthenia gravis, an autoimmune neuromuscular disorder characterized by muscle weakness and fatigue, is seeing increased demand for effective diagnostics and treatments due to rising global incidence and improved healthcare access
- The market is fueled by advancements in immunosuppressive therapies, biologics, and minimally invasive surgical techniques, alongside growing patient awareness and early diagnosis initiatives
- North America dominated the myasthenia gravis disease market with the largest revenue share of 42.5% in 2024, driven by advanced healthcare infrastructure, high adoption of novel therapies, and the presence of key market players. The U.S. leads in research and development, with significant investments in biologics and clinical trials
- Asia-Pacific is expected to be the fastest-growing region during the forecast period, attributed to increasing healthcare investments, rising awareness, and growing prevalence of autoimmune disorders due to urbanization and lifestyle changes
- The blood test segment dominated the largest market revenue share of 38.5% in 2024, driven by its high sensitivity in detecting acetylcholine receptor antibodies, which are present in approximately 85-90% of patients with generalized myasthenia gravis
Report Scope and Myasthenia Gravis Disease Market Segmentation
|
Attributes |
Myasthenia Gravis Disease Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Myasthenia Gravis Disease Market Trends
“Increasing Integration of AI and Big Data Analytics”
- The Global Myasthenia Gravis Disease Market is experiencing a notable trend toward the integration of Artificial Intelligence (AI) and Big Data analytics
- These technologies facilitate advanced data processing, offering deeper insights into patient diagnostics, treatment outcomes, and disease progression
- AI-driven platforms are being developed to analyze patient data, such as antibody levels and muscle response patterns, to predict disease exacerbations and tailor personalized treatment plans
- For instances, companies are leveraging AI to optimize immunosuppressive therapy dosages or identify candidates for thymectomy based on historical patient data and real-time health metrics
- This trend enhances the precision and efficiency of myasthenia gravis management, making treatments more appealing to healthcare providers and patients
- AI algorithms can evaluate a wide range of patient behaviors and clinical data, including response to cholinesterase inhibitors, adverse effects of corticosteroids, and patterns of myasthenic crises.
Myasthenia Gravis Disease Market Dynamics
Driver
“Rising Demand for Advanced Diagnostics and Targeted Therapies”
- Growing awareness of myasthenia gravis and the demand for precise diagnostic tools, such as single-fiber electromyography (EMG) and antibody blood tests, are key drivers for the market
- Advanced treatment options, including monoclonal antibodies and complement inhibitors, enhance patient outcomes and quality of life, boosting market growth
- Government initiatives and regulatory approvals, particularly in North America, such as the FDA’s approval of zilucoplan for generalized myasthenia gravis, are accelerating the adoption of novel therapies
- The expansion of healthcare infrastructure and the integration of IoT in medical devices support real-time patient monitoring and faster data-driven decisions
- Pharmaceutical companies are increasingly investing in biologics and immunotherapies as standard or adjunctive treatments to meet patient needs and improve disease management
Restraint/Challenge
“High Cost of Novel Therapies and Data Privacy Concerns”
- The high cost of advanced treatments, such as monoclonal antibodies and plasmapheresis, poses a significant barrier to adoption, particularly in emerging markets
- Implementing diagnostic tools such as single-fiber EMG and integrating them into healthcare systems can be complex and expensive
- Data privacy and security concerns are a major challenge, as patient data collected for AI-driven analytics and remote monitoring is sensitive and subject to breaches or misuse
- The varied regulatory frameworks across countries for data protection and clinical trials create operational challenges for global pharmaceutical companies and healthcare providers
- These factors may limit market growth in regions with high cost sensitivity or stringent data privacy regulations, such as Europe and parts of Asia-Pacific
Myasthenia Gravis Disease market Scope
The market is segmented on the basis of diagnosis, treatment type, route of administration, end users, and distribution channel.
- By Diagnosis
On the basis of diagnosis, the global myasthenia gravis disease market is segmented into edrophonium test, blood test, repetitive nerve stimulation, single-fiber electromyography (EMG), and others. The blood test segment dominated the largest market revenue share of 38.5% in 2024, driven by its high sensitivity in detecting acetylcholine receptor antibodies, which are present in approximately 85-90% of patients with generalized myasthenia gravis. This non-invasive method is widely adopted for accurate and early diagnosis.
The single-fiber electromyography (EMG) segment is expected to witness the fastest growth rate from 2025 to 2032, driven by its superior sensitivity in diagnosing myasthenia gravis, particularly in cases with negative antibody tests. Advancements in electrophysiological techniques and increasing adoption in specialized neurology centers further accelerate its growth.
- By Treatment Type
On the basis of treatment type, the global myasthenia gravis disease market is segmented into cholinesterase inhibitors, corticosteroids, immunosuppressants, plasmapheresis, autologous hematopoietic stem cell transplantation (HSCT), surgery, and others. The cholinesterase inhibitors segment is expected to hold the largest market revenue share of 32.5% in 2024, primarily due to their widespread use as first-line symptomatic treatment, improving neuromuscular transmission with drugs such as pyridostigmine.
The immunosuppressants segment is anticipated to experience the fastest growth rate of 12.8% from 2025 to 2032, fueled by the rising adoption of targeted therapies, such as monoclonal antibodies, and advancements in biologics that offer improved efficacy and reduced side effects compared to traditional corticosteroids.
- By Route of Administration
On the basis of route of administration, the global myasthenia gravis disease market is segmented into oral and parenteral. The oral segment is expected to hold the largest market revenue share of 68.5% in 2024, driven by the convenience and widespread use of oral medications such as cholinesterase inhibitors and corticosteroids for long-term symptom management.
The parenteral segment is expected to witness the fastest growth rate of 14.2% from 2025 to 2032, propelled by the increasing use of intravenous therapies such as plasmapheresis, intravenous immunoglobulin (IVIg), and novel monoclonal antibodies for severe cases and myasthenic crises.
- By End Users
On the basis of end users, the global myasthenia gravis disease market is segmented into hospitals, homecare, specialty clinics, and others. The hospitals segment is expected to hold the largest market revenue share of 45.5% in 2024, driven by the availability of advanced diagnostic tools, specialized neurology departments, and comprehensive treatment options for managing acute exacerbations and myasthenic crises.
The specialty clinics segment is anticipated to witness robust growth from 2025 to 2032, fueled by the increasing preference for specialized care in neuromuscular disorders, offering tailored treatment plans and access to cutting-edge therapies such as biologics.
- By Distribution Channel
On the basis of distribution channel, the global myasthenia gravis disease market is segmented into hospital pharmacy, online pharmacy, and retail pharmacy. The hospital pharmacy segment is expected to hold the largest market revenue share of 52.5% in 2024, owing to the high demand for specialized medications, including parenteral therapies and biologics, which are primarily dispensed through hospital settings.
The online pharmacy segment is expected to witness the fastest growth rate of 16.5% from 2025 to 2032, driven by the increasing adoption of e-commerce platforms for prescription medications, offering convenience, competitive pricing, and improved access in remote areas.
Myasthenia Gravis Disease Market Regional Analysis
- North America dominated the myasthenia gravis disease market with the largest revenue share of 42.5% in 2024, driven by advanced healthcare infrastructure, high adoption of novel therapies, and the presence of key market players. The U.S. leads in research and development, with significant investments in biologics and clinical trials
- Patients and healthcare providers prioritize innovative treatments such as immunosuppressive therapies, biologics, and thymectomy procedures to manage symptoms and improve quality of life, particularly in regions with aging populations
- Market growth is supported by advancements in diagnostic techniques, such as electromyography and antibody testing, alongside increasing adoption of novel therapeutics in both hospital and outpatient settings
U.S. Myasthenia Gravis Disease Market Insight
The U.S. myasthenia gravis disease market captured the largest revenue share of 89.9% in 2024 within North America, fueled by strong healthcare spending, widespread awareness of autoimmune disorders, and access to cutting-edge treatments. The trend towards personalized medicine and increasing regulatory approvals for biologics, such as eculizumab and rituximab, further boost market expansion. Collaborations between pharmaceutical companies and research institutes complement treatment adoption, creating a robust ecosystem.
Europe Myasthenia Gravis Disease Market Insight
The European myasthenia gravis disease market is expected to witness significant growth, supported by a strong emphasis on healthcare innovation and patient-centric care. Patients seek advanced therapies that improve muscle strength and reduce fatigue while ensuring safety. Growth is prominent in both clinical trials and treatment adoption, with countries such as Germany and France showing significant uptake due to rising autoimmune disease prevalence and supportive reimbursement policies.
U.K. Myasthenia Gravis Disease Market Insight
The U.K. market for myasthenia gravis disease is expected to witness rapid growth, driven by increasing demand for effective symptom management and improved quality of life in urban and rural settings. Growing awareness of diagnostic advancements and therapeutic options encourages adoption. Evolving healthcare policies and guidelines influence treatment choices, balancing efficacy with accessibility.
Germany Myasthenia Gravis Disease Market Insight
Germany is expected to witness rapid growth in the myasthenia gravis disease market, attributed to its advanced healthcare system and high focus on patient outcomes and therapeutic innovation. German patients prefer biologics and targeted therapies that enhance muscle function and contribute to long-term disease management. The integration of these treatments in specialized clinics and research-driven hospitals supports sustained market growth.
Asia-Pacific Myasthenia Gravis Disease Market Insight
The Asia-Pacific region is expected to witness the fastest growth rate, driven by expanding healthcare access, rising autoimmune disease diagnoses, and increasing disposable incomes in countries such as China, India, and Japan. Growing awareness of symptom management, early diagnosis, and advanced therapies is boosting demand. Government initiatives promoting healthcare modernization and disease awareness further encourage the adoption of novel treatments.
Japan Myasthenia Gravis Disease Market Insight
Japan’s myasthenia gravis disease market is expected to witness rapid growth due to strong consumer preference for high-quality, technologically advanced therapies that enhance patient outcomes and safety. The presence of leading pharmaceutical manufacturers and integration of biologics in treatment protocols accelerate market penetration. Rising interest in personalized medicine also contributes to growth.
China Myasthenia Gravis Disease Market Insight
China holds the largest share of the Asia-Pacific myasthenia gravis disease market, propelled by rapid urbanization, rising healthcare investments, and increasing demand for autoimmune disease solutions. The country’s growing middle class and focus on healthcare innovation support the adoption of advanced therapies. Strong domestic research capabilities and competitive pricing enhance market accessibility.
Myasthenia Gravis Disease Market Share
The myasthenia gravis disease industry is primarily led by well-established companies, including:
- Alexion Pharmaceuticals, Inc. (U.S.)
- Bausch Health Companies Inc. (Canada)
- Novartis AG (Switzerland)
- Amneal Pharmaceuticals LLC (U.S.)
- Zydus Group (India)
- Avadel (Ireland)
- Dr. Reddy’s Laboratories Ltd (India)
- Fresenius Kabi AG (Germany)
- Endo Inc. (U.S.)
- Amphastar Pharmaceuticals, Inc. (U.S.)
- Viatris Inc. (U.S.)
- Aurobindo Pharma (India)
- Pfizer Inc. (U.S.)
- Astellas Pharma Inc. (Japan)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Alkem (India)
- Hikma Pharmaceuticals PLC (U.K.)
What are the Recent Developments in Global Myasthenia Gravis Disease Market?
- In April 2025, Johnson & Johnson received FDA approval for IMAAVY (nipocalimab-aahu), a new FcRn blocker designed to treat generalized myasthenia gravis (gMG). This marks the first FcRn inhibitor approved for both anti-acetylcholine receptor (AChR) and anti-muscle-specific kinase (MuSK) antibody-positive patients aged 12 and older. The approval was based on findings from the Vivacity-MG3 Phase 3 trial, which demonstrated significant symptom improvement compared to placebo. IMAAVY™ is expected to be available in May 2025, offering long-lasting disease control and expanding treatment options for gMG patients
- In April 2025, The National Pharmaceutical Regulatory Agency (NPRA) in Malaysia issued a directive requiring all registration holders of statin-containing products to update their local package inserts and consumer medication information leaflets. This update reflects the potential risk of statins inducing or aggravating myasthenia gravis (MG), an autoimmune disorder characterized by muscle weakness and fatigue. The directive applies to 186 registered statin products, including atorvastatin, simvastatin, rosuvastatin, lovastatin, pravastatin, and pitavastatin
- In January 2025, Cartesian Therapeutics, Inc. received FDA approval under the Special Protocol Assessment (SPA) process for its Phase 3 AURORA trial of Descartes-08, an mRNA cell therapy candidate for myasthenia gravis (MG). This agreement confirms that the trial design meets regulatory standards to support a future Biologics License Application (BLA), pending trial results. The randomized, double-blind, placebo-controlled study will evaluate Descartes-08’s efficacy in approximately 100 participants, focusing on MG-ADL score improvements
- In October 2023, UCB received FDA approval for ZILBRYSQ® (zilucoplan), a once-daily subcutaneous C5 complement inhibitor for generalized myasthenia gravis (gMG) in anti-acetylcholine receptor (AChR) antibody-positive adults. This approval followed the Phase 3 RAISE study, which demonstrated statistically significant symptom improvements compared to placebo. With ZILBRYSQ and RYSTIGGO, UCB became the first company to offer two distinct targeted therapies for gMG
- In June 2023, UCB received FDA approval for RYSTIGGO (rozanolixizumab-noli), a subcutaneous monoclonal antibody designed to treat generalized myasthenia gravis (gMG) in adults who are anti-acetylcholine receptor (AChR) or anti-muscle-specific tyrosine kinase (MuSK) antibody positive. This approval was based on findings from the Phase 3 MycarinG study, which demonstrated statistically significant improvements in daily activities such as breathing, talking, and swallowing. RYSTIGGO® is the first FDA-approved treatment for both AChR and MuSK antibody-positive gMG patients
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL MYASTHENIA GRAVIS DISEASE MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL MYASTHENIA GRAVIS DISEASE MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10 PIPELINE ANALYSIS
10.1 CLINICAL TRIALS AND PHASE ANALYSIS
10.2 DRUG THERAPY PIPELINE
10.3 PHASE III CANDIDATES
10.4 PHASE II CANDIDATES
10.5 PHASE I CANDIDATES
10.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR GLOBAL MYASTHENIA GRAVIS DISEASE
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR GLOBAL MYASTHENIA GRAVIS DISEASE MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
11 MARKETED DRUG ANALYSIS
11.1 DRUG
11.1.1 BRAND NAME
11.1.2 GENERICS NAME
11.2 THERAPEUTIC INDIACTION
11.3 PHARACOLOGICAL CLASS OD THE DRUG
11.4 DRUG PRIMARY INDICATION
11.5 MARKET STATUS
11.6 MEDICATION TYPE
11.7 DRUG DOSAGES FORM
11.8 DOSAGES AVAILABILITY
11.9 DRUG ROUTE OF ADMINISTRATION
11.1 DOSING FREQUENCY
11.11 DRUG INSIGHT
11.12 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
11.12.1 FORECAST MARKET OUTLOOK
11.12.2 CROSS COMPETITION
11.12.3 THERAPEUTIC PORTFOLIO
11.12.4 CURRENT DEVELOPMENT SCENARIO
12 MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13 R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14 MARKET OVERVIEW
14.1 DRIVERS
14.2 RESTRAINTS
14.3 OPPORTUNITIES
14.4 CHALLENGES
15 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY DISEASE TYPE
15.1 OVERVIEW
15.2 OCULAR MYASTHENIA GRAVIS
15.3 GENERALIZED MYASTHENIA GRAVIS
15.3.1 MILD GENERALIZED
15.3.2 MODERATE GENERALIZED
15.3.3 SEVERE GENERALIZED
15.4 CONGENITAL MYASTHENIC SYNDROMES (CMS)
15.5 TRANSIENT NEONATAL MYASTHENIA GRAVIS
15.6 JUVENILE MYASTHENIA GRAVIS
16 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY TREATMENT TYPE
16.1 OVERVIEW
16.2 MEDICATION
16.2.1 CHOLINESTERASE INHIBITORS
16.2.1.1. PYRIDOSTIGMINE
16.2.1.2. NEOSTIGMINE
16.2.2 IMMUNOSUPPRESSANTS
16.2.2.1. CORTICOSTEROIDS (E.G., PREDNISONE)
16.2.2.2. NON-STEROIDAL IMMUNOSUPPRESSANTS
16.2.2.2.1. AZATHIOPRINE
16.2.2.2.2. OTHERS
16.2.3 MONOCLONAL ANTIBODIES
16.2.3.1. ANTI-CD20 MONOCLONAL ANTIBODIES (E.G., RITUXIMAB)
16.2.3.2. COMPLEMENT INHIBITORS
16.2.3.2.1. ECULIZUMAB
16.2.3.2.2. OTHERS
16.2.3.3. FCRN ANTAGONISTS (E.G., EFGARTIGIMOD)
16.2.4 EMERGING DRUGS
16.3 SURGICAL TREATMENT
16.3.1 THYMECTOMY
16.3.1.1. OPEN THYMECTOMY
16.3.1.2. MINIMALLY INVASIVE THYMECTOMY
16.4 PLASMAPHERESIS AND INTRAVENOUS IMMUNOGLOBULIN (IVIG)
16.4.1 PLASMAPHERESIS
16.4.2 IVIG
16.4.2.1. POLYCLONAL IVIG
16.4.2.2. SUBCUTANEOUS IVIG
16.5 EMERGING THERAPIES
16.5.1 GENE THERAPY
16.5.2 CELL THERAPY
16.5.3 TARGETED IMMUNOTHERAPIES
17 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY DIAGNOSIS
17.1 OVERVIEW
17.2 EDROPHONIUM TEST
17.3 BLOOD TEST
17.4 REPETITIVE NERVE STIMULATION
17.5 SINGLE-FIBER ELECTROMYOGRAPHY (EMG)
17.6 OTHERS
18 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY ROUTE OF ADMINISTRATION
18.1 OVERVIEW
18.2 ORAL
18.3 PARENTERAL
18.4 SUBCUTANEOUS
18.5 OTHERS
19 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY DOSAGE FORM
19.1 OVERVIEW
19.2 TABLET
19.3 CAPSULE
19.4 INJECTABLE
19.5 OTHER
20 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY AGE GROUP
20.1 OVERVIEW
20.2 PEDIATRIC
20.3 ADULTS
20.4 GERIATRIC
21 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY GENDER
21.1 OVERVIEW
21.2 MALE
21.3 FEMALE
22 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY END USER
22.1 OVERVIEW
22.2 HOSPITALS
22.2.1 GENERAL HOSPITALS
22.2.2 SPECIALTY HOSPITALS
22.3 CLINICS
22.3.1 NEUROLOGY CLINICS
22.3.2 SPECIALTY MYASTHENIA GRAVIS TREATMENT CENTERS
22.4 HOMECARE SETTINGS
22.5 RESEARCH AND ACADEMIC INSTITUTES
23 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY DISTRIBUTION CHANNEL
23.1 OVERVIEW
23.2 HOSPITAL
23.3 PHARMACIES
23.4 RETAIL PHARMACIES
23.5 ONLINE PHARMACIES
23.6 SPECIALTY PHARMACIES
24 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, SWOT AND DBMR ANALYSIS
25 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: GLOBAL
25.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.3 COMPANY SHARE ANALYSIS: EUROPE
25.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
25.5 MERGERS & ACQUISITIONS
25.6 NEW PRODUCT DEVELOPMENT & APPROVALS
25.7 EXPANSIONS
25.8 REGULATORY CHANGES
25.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, BY REGION
GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
26.1 NORTH AMERICA
26.1.1 U.S.
26.1.2 CANADA
26.1.3 MEXICO
26.2 EUROPE
26.2.1 GERMANY
26.2.2 U.K.
26.2.3 ITALY
26.2.4 FRANCE
26.2.5 SPAIN
26.2.6 RUSSIA
26.2.7 SWITZERLAND
26.2.8 TURKEY
26.2.9 BELGIUM
26.2.10 NETHERLANDS
26.2.11 DENMARK
26.2.12 SWEDEN
26.2.13 POLAND
26.2.14 NORWAY
26.2.15 FINLAND
26.2.16 REST OF EUROPE
26.3 ASIA-PACIFIC
26.3.1 JAPAN
26.3.2 CHINA
26.3.3 SOUTH KOREA
26.3.4 INDIA
26.3.5 SINGAPORE
26.3.6 THAILAND
26.3.7 INDONESIA
26.3.8 MALAYSIA
26.3.9 PHILIPPINES
26.3.10 AUSTRALIA
26.3.11 NEW ZEALAND
26.3.12 VIETNAM
26.3.13 TAIWAN
26.3.14 REST OF ASIA-PACIFIC
26.4 SOUTH AMERICA
26.4.1 BRAZIL
26.4.2 ARGENTINA
26.4.3 REST OF SOUTH AMERICA
26.5 MIDDLE EAST AND AFRICA
26.5.1 SOUTH AFRICA
26.5.2 EGYPT
26.5.3 BAHRAIN
26.5.4 UNITED ARAB EMIRATES
26.5.5 KUWAIT
26.5.6 OMAN
26.5.7 QATAR
26.5.8 SAUDI ARABIA
26.5.9 REST OF MEA
26.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
27 GLOBAL MYASTHENIA GRAVIS DISEASE MARKET, COMPANY PROFILE
27.1 ALEXION PHARMACEUTICALS
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 ARGENX
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 UCB PHARMA
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 IMMUNOVANT
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 JANSSEN PHARMACEUTICALS (JOHNSON & JOHNSON)
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 HORIZON THERAPEUTICS
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 CATALYST PHARMACEUTICALS
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 TG THERAPEUTICS
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 MITSUBISHI TANABE PHARMA CORPORATION
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.1 APELLIS PHARMACEUTICALS
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 NOVARTIS
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 ASTELLAS PHARMA
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 ADAMIS PHARMACEUTICALS CORPORATION
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 CURAVAC
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 SHANGHAI PHARMACEUTICALS
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 BIOHAVEN PHARMACEUTICALS
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
28 RELATED REPORTS
29 CONCLUSION
30 QUESTIONNAIRE
31 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.