Global Myasthenia Gravis Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
2.64 Billion
USD
5.31 Billion
2024
2032
USD
2.64 Billion
USD
5.31 Billion
2024
2032
| 2025 –2032 | |
| USD 2.64 Billion | |
| USD 5.31 Billion | |
|
|
|
|
Myasthenia Gravis Treatment Market Size
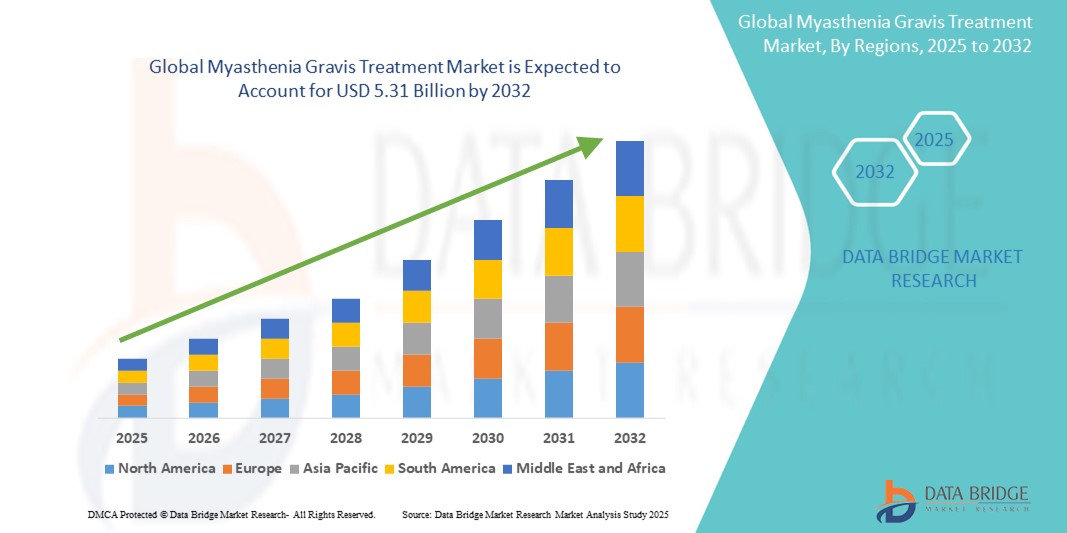
- The global myasthenia gravis treatment market size was valued at USD 2.64 billion in 2024 and is expected to reach USD 5.31 billion by 2032, at a CAGR of 9.10% during the forecast period
- The market growth is largely fueled by the increasing prevalence of autoimmune neuromuscular disorders and the growing awareness of Myasthenia Gravis (MG), which have led to heightened demand for early diagnosis and effective treatment options. Advances in biotechnology and immunology are significantly transforming the treatment landscape for MG, particularly through the development of monoclonal antibodies, complement inhibitors, and targeted immunotherapies
- Furthermore, rising patient preference for minimally invasive and targeted therapies, as well as an increase in clinical trials and regulatory approvals for innovative biologics, are accelerating the uptake of myasthenia gravis treatment solutions. These converging factors are significantly boosting the industry's growth, as stakeholders invest in R&D, personalized medicine, and global expansion strategies
Myasthenia Gravis Treatment Market Analysis
- Myasthenia gravis treatment options are becoming increasingly essential due to the rising prevalence of autoimmune neuromuscular disorders globally, with growing awareness and early diagnosis significantly boosting treatment adoption across healthcare systems
- The escalating demand for advanced therapies is driven by the growing incidence of generalized myasthenia gravis (gMG), increasing approvals of monoclonal antibodies and complement inhibitors, and improved access to neurology care
- North America dominated the myasthenia gravis treatment market with the largest revenue share of 41.6% in 2024, attributed to robust healthcare infrastructure, early adoption of biologics and novel therapies, and strong presence of leading pharmaceutical companies. The U.S. is experiencing substantial growth in new treatment initiations, driven by clinical advancements and favorable reimbursement policies
- Asia-Pacific is projected to be the fastest growing region in the myasthenia gravis treatment market, registering a CAGR of 13.7% from 2025 to 2032, due to increasing healthcare expenditures, improving access to neurological specialists, and heightened awareness of rare autoimmune diseases
- The proton radiation segment dominated the myasthenia gravis treatment market with a market share of 38.6% in 2024, driven by its superior ability to precisely target tumors while sparing surrounding healthy tissue. Its growing application in treating delicate and localized cancers—such as ocular, brain, and pediatric tumors—has contributed to strong clinical adoption and increasing preference among healthcare providers
Report Scope and Myasthenia Gravis Treatment Market Segmentation
|
Attributes |
Myasthenia Gravis Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Myasthenia Gravis Treatment Market Trends
“Rising Adoption of Targeted Biologic Therapies”
- A significant and accelerating trend in the global myasthenia gravis treatment market is the growing preference for biologic and targeted therapies, particularly monoclonal antibodies and complement inhibitors, which offer improved efficacy in managing moderate to severe cases and treatment-resistant patients
- For instance, eculizumab and ravulizumab, both complement C5 inhibitors, have demonstrated sustained reduction in symptom severity and hospitalizations, especially in patients with refractory generalized myasthenia gravis (gMG). Their success has catalyzed further development of precision therapies in the immunoneurology space
- There is also increasing clinical and commercial momentum behind FcRn inhibitors such as efgartigimod and rozanolixizumab, which work by reducing pathogenic IgG antibodies that contribute to neuromuscular junction dysfunction. These agents offer a novel approach for patients unresponsive to standard cholinesterase inhibitors and corticosteroids
- The integration of newer biologics into treatment guidelines is reshaping how neurologists manage the disease, shifting from broad immunosuppressive regimens to more targeted, patient-specific options with favorable safety profiles
- Moreover, the expansion of clinical trials, real-world evidence data, and increasing FDA and EMA approvals for novel treatments are strengthening physician confidence and expanding access to next-generation therapies
- This shift toward personalized immunotherapy is redefining standards of care for Myasthenia Gravis, creating substantial market opportunities for pharmaceutical innovators and driving sustained investment into autoimmune neurology pipelines
Myasthenia Gravis Treatment Market Dynamics
Driver
“Growing Demand for Effective Neuromuscular Therapies and Innovation in Treatment Modalities”
- The increasing incidence of autoimmune neuromuscular disorders such as myasthenia gravis (MG), along with rising awareness and improved diagnostic capabilities, is a significant driver for the heightened demand for advanced MG treatment solutions
- For instance, in March 2024, argenx SE received FDA approval for Vyvgart Hytrulo, a subcutaneous version of efgartigimod, for generalized myasthenia gravis. This innovative therapy highlights the trend of biopharmaceutical companies developing more convenient and patient-friendly administration routes, contributing to robust market expansion
- As healthcare systems globally place a stronger emphasis on rare and chronic disease management, patients are increasingly opting for targeted treatments such as monoclonal antibodies, complement inhibitors, and corticosteroids to manage symptoms and prevent flare-ups of MG
- Furthermore, the development of precision medicine and ongoing research into genetic and antibody-targeted therapies are establishing advanced treatments as the modern standard for MG care, offering long-term benefits with fewer side effects than traditional options
- The convenience of at-home treatment protocols, subcutaneous administration, and reduced treatment burden, along with increased government and private investments in rare disease therapeutics, are key factors propelling the adoption of innovative MG therapies. The trend towards individualized care plans and the availability of FDA/EMA-approved options further contribute to market growth
Restraint/Challenge
“High Cost of Biologic Therapies and Limited Accessibility in Low-Income Regions”
- The high cost of advanced biologics and immunotherapies for myasthenia gravis remains a substantial barrier to market penetration, especially in emerging economies. These treatments, often requiring long-term administration, impose a heavy financial burden on both patients and healthcare systems
- For instance, complement inhibitors such as eculizumab (Soliris) can cost hundreds of thousands of dollars annually, making them inaccessible to a large segment of the global population lacking insurance or subsidy support
- Addressing these affordability concerns through expanded health coverage, generic or biosimilar alternatives, and government reimbursement programs is crucial for equitable treatment access. Companies like UCB Pharma and Roche are actively investing in global access programs and affordability initiatives to extend the reach of their MG therapeutics
- Moreover, the availability of trained specialists and accurate diagnostic infrastructure remains limited in rural and underserved regions, further restricting timely diagnosis and treatment initiation
- Overcoming these challenges through international healthcare collaborations, investments in diagnostics, and the development of cost-effective MG treatments will be vital for sustained market growth and widespread patient impact
Myasthenia Gravis Treatment Market Scope
The market is segmented on the basis of radiation type, application and end user.
- By Radiation Type
On the basis of radiation type, the myasthenia gravis treatment market is segmented into proton radiation, electron radiation, photon radiation, and carbon-ion radiation. The proton radiation segment accounted for the largest market revenue share of 38.6% in 2024, driven by its superior ability to precisely target tumors while sparing surrounding healthy tissue. Its growing application in treating delicate and localized cancers, including ocular, brain, and pediatric tumors, has contributed to its strong clinical adoption.
The carbon-ion radiation segment is projected to witness the fastest CAGR of 9.8% from 2025 to 2032, owing to its higher relative biological effectiveness (RBE) and potential in treating radioresistant and deep-seated tumors. Increased investments in advanced radiotherapy infrastructure and research collaborations are also accelerating segment growth.
- By Application
On the basis of application, the market is segmented into prostate cancer, lung cancer, breast cancer, brain cancer, gynecological cancers, gastrointestinal cancers, and other cancers. The prostate cancer segment dominated the largest market share of 26.3% in 2024 due to the rising global incidence of prostate malignancies and favorable reimbursement policies for radiotherapy in developed markets. Proton and photon radiation therapies are commonly utilized for their precision and minimized side effects.
The brain cancer segment is expected to witness the highest CAGR of 10.2% over the forecast period, driven by increased utilization of advanced imaging-guided therapies, such as stereotactic radiosurgery (SRS), and the need for treatments that minimize cognitive side effects.
- By End User
On the basis of end user, the myasthenia gravis treatment market is segmented into hospitals, independent radiotherapy centers, and others. The hospitals segment held the largest market revenue share of 61.5% in 2024, attributed to their extensive infrastructure, multi-specialty cancer care offerings, and access to advanced radiotherapy technologies. Hospitals remain the primary setting for both initial diagnosis and treatment execution for most cancer types.
The independent radiotherapy centers segment is projected to register the fastest CAGR of 8.7% from 2025 to 2032, driven by the decentralization of cancer care services, increasing outpatient volumes, and cost-effective service models focused solely on radiation therapy.
Myasthenia Gravis Treatment Market Regional Analysis
- North America dominated the myasthenia gravis treatment market with the largest revenue share of 41.6% in 2024, driven by the high prevalence of autoimmune neuromuscular disorders and increased awareness about rare disease treatment options
- The region benefits from advanced healthcare infrastructure, strong reimbursement frameworks, and widespread availability of FDA-approved treatments such as efgartigimod (Vyvgart) and Soliris, supporting rapid adoption of novel therapies
- Continued clinical research, growing patient advocacy, and favorable government initiatives for rare disease management further bolster market expansion in both the U.S. and Canada
U.S. Myasthenia Gravis Treatment Market Insight
The U.S. myasthenia gravis treatment market insight market captured the largest revenue share of 81% in 2024 within North America, supported by a growing number of clinical trials and increased physician adoption of targeted therapies. The dominance is attributed to the early availability of biologics, increasing diagnosis rates, and high patient awareness, alongside advanced specialty clinics and neurology centers. Furthermore, the presence of key players such as argenx, UCB, and AstraZeneca, who are actively engaged in strategic partnerships and regulatory filings, continues to propel market momentum.
Europe Myasthenia Gravis Treatment Market Insight
The Europe myasthenia gravis treatment market insight market is projected to expand at a significant CAGR during the forecast period, driven by rising MG incidence, improved diagnostic capabilities, and the approval of innovative therapies across the EU. EU nations benefit from centralized regulatory processes via the EMA and growing investments in rare disease infrastructure. The region is also seeing a shift toward outpatient care and home infusion therapy, which increases the accessibility of long-term MG treatment.
U.K. Myasthenia Gravis Treatment Market Insight
The U.K. myasthenia gravis treatment market insight market is anticipated to grow at a notable CAGR during the forecast period, driven by a robust national health system (NHS) that supports access to orphan drugs and rare disease treatments. The presence of well-established MG patient registries and increasing government focus on personalized medicine are enhancing early diagnosis and appropriate treatment plans Partnerships between research institutes and biopharma firms are also fueling development of next-gen immunotherapies in the U.K.
Germany Myasthenia Gravis Treatment Market Insight
The Germany myasthenia gravis treatment market insight market is expected to expand at a considerable CAGR, supported by the country’s leadership in neurology research, reimbursement accessibility, and clinical excellence in treating autoimmune conditions. With a strong emphasis on innovation, Germany is witnessing an increase in clinical trial sites and hospital-based therapy adoption, especially for monoclonal antibodies and complement inhibitors. Increased awareness initiatives and specialized neuromuscular clinics are also contributing to market growth.
Asia-Pacific Myasthenia Gravis Treatment Market Insight
The Asia-Pacific myasthenia gravis treatment market insight market is poised to grow at the fastest CAGR of 13.7% from 2025 to 2032, driven by rapid healthcare infrastructure development, rising MG prevalence, and improved access to biologics in countries like China, Japan, and India. Government efforts toward early diagnosis of rare diseases and growing investments from multinational companies are catalyzing therapeutic expansion. APAC is also emerging as a cost-effective manufacturing hub for MG drugs, facilitating better affordability and wider reach.
Japan Myasthenia Gravis Treatment Market Insight
The Japan myasthenia gravis treatment market insight market is gaining traction due to its strong national rare disease framework, increasing elderly population, and rapid adoption of innovative treatment modalities. Japan’s healthcare system emphasizes precision medicine, and the market is experiencing rising uptake of efgartigimod and other next-gen antibody therapies. With government-funded rare disease programs and supportive pricing mechanisms, Japan continues to be a frontrunner in MG treatment access in Asia.
China Myasthenia Gravis Treatment Market Insight
The China myasthenia gravis treatment market insight market accounted for the largest market revenue share in Asia Pacific in 2024, supported by rising MG awareness, growing middle-class population, and strong domestic pharmaceutical activity. China’s inclusion of more rare disease therapies in national reimbursement lists and fast-track approval pathways have encouraged rapid market entry for novel MG drugs. The development of specialty neurology hospitals and research into biosimilars further strengthens China’s market position.
Myasthenia Gravis Treatment Market Share
The myasthenia gravis treatment industry is primarily led by well-established companies, including:
- Mettler-Toledo (U.S.)
- Varian Medical Systems (U.S.)
- Elekta AB (Sweden)
- Accuray (U.S.)
- Siemens Healthineers AG (Germany)
- IBA Dosimetry (Belgium)
- Sun Nuclear Corporation (U.S.)
- ViewRay (U.S.)
- RaySearch Laboratories (Sweden)
- Mevion Medical Systems (U.S.)
- Hitachi Healthcare (Japan)
- Prowess Inc. (a subsidiary of Altair) (U.S.)
- Brainlab (Germany)
- Koninklijke Philips N.V. (Netherlands)
Latest Developments in Global Myasthenia Gravis Treatment Market
- In April 2025, Johnson & Johnson received FDA approval for IMAAVY (nipocalimab‑aahu), a first-in-class FcRn blocker for the treatment of generalized myasthenia gravis (gMG) in adults and adolescents aged 12 years and older. This marks the broadest patient indication in the FcRn class to date, covering both anti-AChR and anti-MuSK antibody-positive patients. The approval strengthens J&J’s leadership in neuroimmunology and expands its rare disease portfolio
- In June 2023, argenx received FDA approval for VYVGART Hytrulo, the first subcutaneous injectable FcRn blocker, for adult anti-AChR antibody-positive gMG patients. This advancement allows patients to self-administer treatment at home, providing more flexibility and convenience in disease management. The launch has accelerated the shift toward patient-centric biologics in neuromuscular care
- In October 2023, UCB secured FDA approval for ZILBRYSQ (zilucoplan), a once-daily subcutaneous peptide inhibitor of complement C5, for adult patients with anti-AChR antibody-positive gMG. ZILBRYSQ offers the first and only self-administered complement inhibitor, positioning UCB as a leading player in complement-mediated neuromuscular diseases
- In June 2023, UCB also received FDA approval for RYSTIGGO (rozanolixizumab-noli), an FcRn-blocking monoclonal antibody for both AChR and MuSK-positive gMG patients. Administered subcutaneously on a weekly basis, RYSTIGGO offers a targeted mechanism with broad coverage and contributes to personalized therapy strategies in gMG treatment
- In March 2024, Meiji Seika Pharma and its subsidiary Medicago announced their collaboration with Japanese neurology centers to expand access to investigational gMG biologics, focusing on precision therapy and biomarker-driven clinical trials. This initiative is expected to enhance treatment options for MuSK-positive and seronegative myasthenia gravis patients in Asia
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.