Global Myeloproliferative Disorders Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
8.50 Billion
USD
11.19 Billion
2021
2029
USD
8.50 Billion
USD
11.19 Billion
2021
2029
| 2022 –2029 | |
| USD 8.50 Billion | |
| USD 11.19 Billion | |
|
|
|
|
Myeloproliferative Disorders Drugs Market Analysis and Size
The myeloproliferative disorders drugs market is expected to witness significant growth during the forecast period. The U.S. dominated played at the forefront of the seven major markets. The wide availability of novel drugs combined with the presence of a strong pipeline is one of the major trends fuelling market growth. The prevalence rates of myeloproliferative disorders are the highest in North America and Western Europe and the lowest in East-Asian countries. COVID-19 also had a major impact on the market growth.
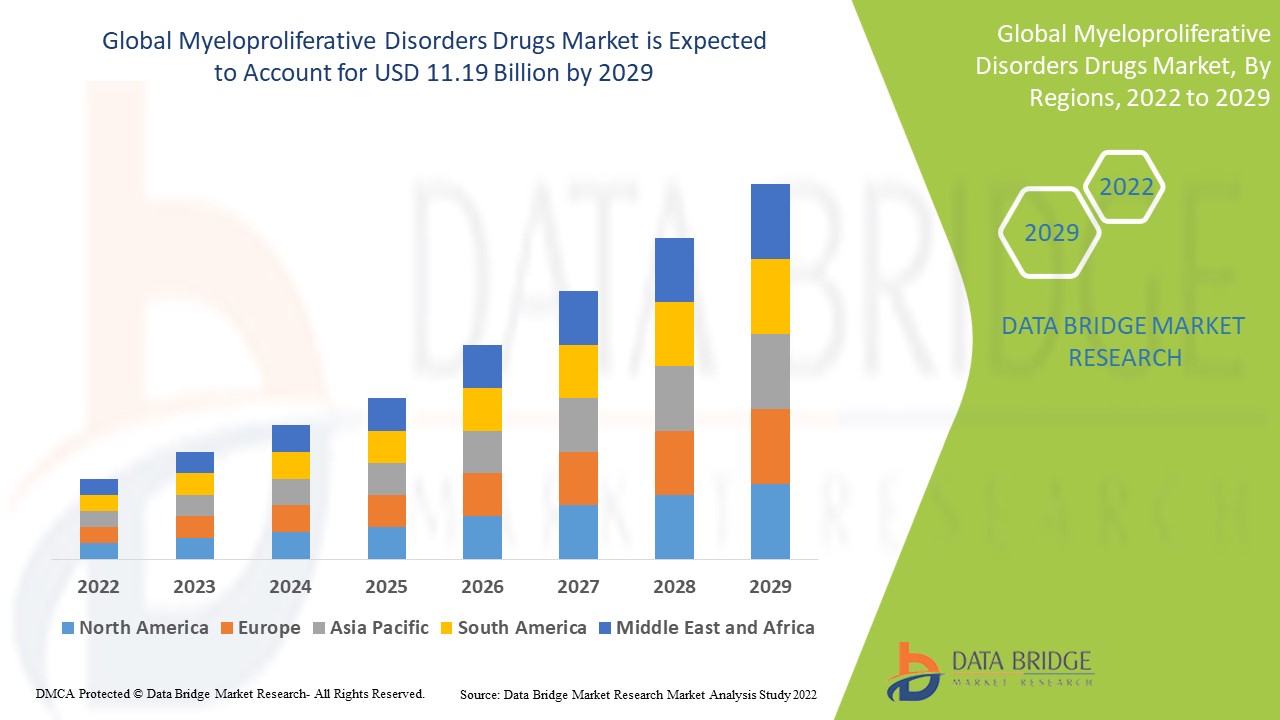
Data Bridge Market Research analyses a growth rate in the myeloproliferative disorders drugs market in the forecast period 2022-2029. The expected CAGR of myeloproliferative disorders drugs market is tend to be around 3.50% in the mentioned forecast period. The market was valued at USD 8.50 billion in 2021, and it would grow upto USD 11.19 billion by 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Myeloproliferative Disorders Drugs Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Philadelphia Chromosome–Negative Myeloproliferative Neoplasms, Philadelphia Chromosome–Positive Chronic Myeloid Leukemia), Treatment Type (Targeted Therapy, Chemotherapy, Others), Route of Administration (Oral, Parenteral, Others), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Pfizer Inc (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Mylan N.V. (U.S.), Fresenius Kabi AG (Germany), Hikma Pharmaceuticals PLC (U.K.), Novartis AG (Switzerland), Teva Pharmaceutical Industries Ltd. (Israel), Bristol Myers Squibb Company (U.S.) GSK Plc. (U.K.), Bayer AG (Germany), Sun Pharmaceutical Industries Ltd (India), Boehringer Ingelheim International Gmbh (Germany), Sanofi (France) |
|
Market Opportunities |
|
Market Definition
Myeloproliferative disorders are the type of haematological disorders of bone marrow and blood. In this disease, abnormal cells grow from the bone marrow. It occurs when scar tissue gathers inside the bone marrow which results in insufficient production of the blood cells. There are numerous types of myeloproliferative neoplasmsnamely chronic myelogenous leukemia, polycythemia vera, essential thrombocythemia, and others. These conditions are developed slowly over a period of time, and most of the people are diagnosed after the age of 60 years.
Global Myeloproliferative Disorders Drugs Market Dynamics
Drivers
- Increased Awareness Associated With Chemotherapy
The market growth can be attributed to the wide availability of agents required for chemotherapy and the approval of Ruxolitinib (Jakafi) by the US FDA. It is a JAK1 and JAK2 inhibitor that targets overactive JAK pathway signalling, which plays a main role in developing myelofibrosis. Presently, Ruxolitinib is the only FDA-approved chemotherapeutic agent for the treatment of myelofibrosis, which in return is boosting the demand for this drug and assisting the growth of the segment.
- Increasing Incidence in Elderly Population
Myelofibrosis cases are mostly witnessed in the age group ≤ 40 years, 41−60 years, and > 60 years. The >60 years group saw highest number of patients mostly around 10,275 in 2021 in the U.S. In 2021, eligible treatment population of myelofibrosis accounted for 9,511 cases in the U.S. Thus, this elderly population with higher chances of getting this disease boosts the market growth.
Opportunities
- Rising R&D and New Drug Approvals
The unmet needs of patients who are suffering from myelofibrosis are encouraging manufacturers to innovate new solutions. The increasing investments in the R&D activities to develop a precise treatment for myelofibrosis is anticipated to boost the growth of the industry in the forecast period. The speedy technological advancements and ongoing clinical trials contribute to industry growth. Several other factors such as improved healthcare sector, reimbursement policies, favourable government initiatives, busy lifestyle and changing dietary patterns are also leading to the industry growth.
- Increasing Collaborations and Market Expansion
Some of the bulging companies operating in the market are Novartis, Pfizer, Takeda, Incyte, Bristol-Myers Squibb, and Teva. Collaborations for development, broader product portfolios, and regional expansion in developing markets are the major strategic undertakings of these market players to increase their market share. Thus, these factors are growing the market.
Restraints/Challenges
- Lack of Awareness
The lack of awareness about these disorders and the unavailability of numerous awareness programs hamper the market growth. The available drugs have not shown disease-modifying activity and are still mostly inadequate for managing major unmet needs.
- High Cost
The huge expenditure associated with these agents hamper the market growth. The huge drug development and associated processes hamper the market's growth.
This global myeloproliferative disorders drugs market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global myeloproliferative disorders drugs market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In April 2020, Janssen Pharmaceutical Company which is a subsidiary of Johnson & Johnson, received the U.S. FDA approval for IMBRUVICA (ibrutinib) combined with rituximab used for the treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
Global Myeloproliferative Disorders Drugs Market Scope
The global myeloproliferative disorders drugs market is segmented on the basis of type, treatment type, route of administration, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Philadelphia Chromosome–Negative Myeloproliferative Neoplasms
- Philadelphia Chromosome–Positive Chronic Myeloid Leukemia
Treatment Type
- Chemotherapy
- Targeted Therapy
- Others
Route of Administration
- Oral
- Parenteral
- Others
End User
- Hospitals
- Homecare
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Myeloproliferative Disorders Drugs Market Regional Analysis/Insights
The global myeloproliferative disorders drugs market is analyzed and market size insights and trends are provided by type, treatment type, route of administration, distribution channel and end-user as referenced above.
The major countries covered in the global myeloproliferative disorders drugs market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Asia-Pacific has been witnessing positive growth for global myeloproliferative disorders drugs market throughout the forecasted period due to the increased prevalence of myeloproliferative disorders, high demand for targeted therapies, and advanced healthcare facilities
North America dominates the market due to increasing disease awareness and rapidly disposable income.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Myeloproliferative Disorders Drugs Market Share Analysis
The global myeloproliferative disorders drugs market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global myeloproliferative disorders drugs market.
Key players operating in the global myeloproliferative disorders drugs market include:
- Pfizer Inc (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Mylan N.V. (U.S.)
- Fresenius Kabi AG (Germany)
- Hikma Pharmaceuticals PLC (U.K.)
- Novartis AG (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Bristol Myers Squibb Company (U.S.)
- GSK Plc. (U.K.)
- Bayer AG (Germany)
- Sun Pharmaceutical Industries Ltd (India)
- Boehringer Ingelheim International Gmbh (Germany)
- Sanofi (France)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL XX SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR XX
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
10.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.13.1 FORECAST MARKET OUTLOOK
10.13.2 CROSS COMPETITION
10.13.3 THERAPEUTIC PORTFOLIO
10.13.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 MARKET OVERVIEW
13.1 DRIVERS
13.2 RESTRAINTS
13.3 OPPORTUNITIES
13.4 CHALLENGES
14 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY TYPE
14.1 OVERVIEW
14.2 PHILADELPHIA CHROMOSOME (PH)-NEGATIVE MYELOPROLIFERATIVE DISORDERS (MPDS)
14.2.1 ESSENTIAL THROMBOCYTHEMIA (ET)
14.2.2 POLYCYTHEMIA VERA (PV)
14.2.3 IDIOPATHIC MYELOFIBROSIS (IMF)
14.3 PHILADELPHIA CHROMOSOME–POSITIVE CHRONIC MYELOID LEUKEMIA
14.3.1 BY PHASES
14.3.1.1. CHRONIC PHASE
14.3.1.2. ACCELERATED PHASE
14.3.1.3. BLAST PHASE
15 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY TREATMENT TYPE
15.1 OVERVIEW
15.2 TARGETED THERAPY
15.2.1 JAK INHIBITORS
15.2.1.1. RUXOLITINIB
15.2.1.2. FEDRATINIB
15.2.2 TYROSINE KINASE INHIBITOR
15.2.2.1. IMATINIB
15.2.2.2. NILOTINIB
15.2.2.3. BOSUTINIB
15.2.2.4. DASATINIB
15.2.2.5. BAFETINIB
15.2.2.6. PACRITINIB
15.2.2.7. OTHERS
15.2.3 THALIDOMIDE
15.2.4 OTHERS
15.3 CHEMOTHERAPY
15.3.1 HYDROXYUREA
15.3.2 CYTARABINE
15.3.3 ANTHRACYCLINES
15.3.4 ALKYLATING AGENTS
15.3.4.1. BUSULFAN
15.3.4.2. MELPHALAN
15.3.5 AZACITIDINE
15.3.6 OTHERS
15.4 OTHERS
15.4.1 ANAGRELIDE
15.4.2 DANAZOL
15.4.3 LENALIDOMIDE
15.4.4 INTERFERON ALPHA
15.4.5 ARSENIC TRIOXIDE
15.4.6 OTHERS
16 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY ROUTE OF ADMINISTRATION
16.1 OVERVIEW
16.2 ORAL
16.2.1 TABLETS
16.2.2 CAPSULES
16.3 PARENTERAL
16.3.1 INTRAMUSCULAR
16.3.2 INTRAVENOUS
16.3.3 SUBCUTANEOUS
16.3.4 OTHERS (IF ANY)
17 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY DRUG TYPE
17.1 OVERVIEW
17.2 BRANDED
17.2.1 GLEEVEC
17.2.2 SPRYCEL
17.2.3 TASIGNA
17.2.4 JAKAFI
17.2.5 THALOMID
17.2.6 AGRYLIN
17.2.7 SIKLOS
17.2.8 INREBIC
17.2.9 DROXIA
17.2.10 SPRYCEL
17.2.11 OTHERS
17.3 GENERIC
18 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY MODE OF PURCHASE
18.1 OVERVIEW
18.2 PRESCRIPTION
18.3 OVER THE COUNTER (OTC)
19 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY POPULATION TYPE
19.1 OVERVIEW
19.2 PEDIATRIC
19.2.1 BY TYPE
19.2.1.1. PHILADELPHIA CHROMOSOME (PH)-NEGATIVE MYELOPROLIFERATIVE DISORDERS (MPDS)
19.2.1.1.1. ESSENTIAL THROMBOCYTHEMIA (ET)
19.2.1.1.2. POLYCYTHEMIA VERA (PV)
19.2.1.1.3. IDIOPATHIC MYELOFIBROSIS (IMF)
19.2.1.2. PHILADELPHIA CHROMOSOME–POSITIVE CHRONIC MYELOID LEUKEMIA
19.3 ADULTS
19.3.1 BY TYPE
19.3.1.1. PHILADELPHIA CHROMOSOME (PH)-NEGATIVE MYELOPROLIFERATIVE DISORDERS (MPDS)
19.3.1.1.1. ESSENTIAL THROMBOCYTHEMIA (ET)
19.3.1.1.2. POLYCYTHEMIA VERA (PV)
19.3.1.1.3. IDIOPATHIC MYELOFIBROSIS (IMF)
19.3.1.2. PHILADELPHIA CHROMOSOME–POSITIVE CHRONIC MYELOID LEUKEMIA
19.4 GERIATRIC
19.4.1 BY TYPE
19.4.1.1. PHILADELPHIA CHROMOSOME (PH)-NEGATIVE MYELOPROLIFERATIVE DISORDERS (MPDS)
19.4.1.1.1. ESSENTIAL THROMBOCYTHEMIA (ET)
19.4.1.1.2. POLYCYTHEMIA VERA (PV)
19.4.1.1.3. IDIOPATHIC MYELOFIBROSIS (IMF)
19.4.1.2. PHILADELPHIA CHROMOSOME–POSITIVE CHRONIC MYELOID LEUKEMIA
20 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.2.1 BY TYPE
20.2.1.1. PRIVATE
20.2.1.2. PUBLIC
20.3 SPECIALTY CLINICS
20.4 DIAGNOSTICS CENTERS
20.5 RESEARCH INSTITUTES
20.6 HOMECARE SETTINGS
20.7 OTHERS
21 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 HOSPITAL PHARMACY
21.3 ONLINE PHARMACY
21.4 RETAIL PHARMACY
22 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, SWOT AND DBMR ANALYSIS
23 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: GLOBAL
23.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS: EUROPE
23.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.5 MERGERS & ACQUISITIONS
23.6 NEW PRODUCT DEVELOPMENT & APPROVALS
23.7 EXPANSIONS
23.8 REGULATORY CHANGES
23.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, BY REGION
24.1 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.2 NORTH AMERICA
24.2.1 U.S.
24.2.2 CANADA
24.2.3 MEXICO
24.3 EUROPE
24.3.1 GERMANY
24.3.2 U.K.
24.3.3 ITALY
24.3.4 FRANCE
24.3.5 SPAIN
24.3.6 RUSSIA
24.3.7 SWITZERLAND
24.3.8 TURKEY
24.3.9 BELGIUM
24.3.10 NETHERLANDS
24.3.11 DENMARK
24.3.12 SWEDEN
24.3.13 POLAND
24.3.14 NORWAY
24.3.15 FINLAND
24.3.16 REST OF EUROPE
24.4 ASIA-PACIFIC
24.4.1 JAPAN
24.4.2 CHINA
24.4.3 SOUTH KOREA
24.4.4 INDIA
24.4.5 SINGAPORE
24.4.6 THAILAND
24.4.7 INDONESIA
24.4.8 MALAYSIA
24.4.9 PHILIPPINES
24.4.10 AUSTRALIA
24.4.11 NEW ZEALAND
24.4.12 VIETNAM
24.4.13 TAIWAN
24.4.14 REST OF ASIA-PACIFIC
24.5 SOUTH AMERICA
24.5.1 BRAZIL
24.5.2 ARGENTINA
24.5.3 REST OF SOUTH AMERICA
24.6 MIDDLE EAST AND AFRICA
24.6.1 SOUTH AFRICA
24.6.2 EGYPT
24.6.3 BAHRAIN
24.6.4 UNITED ARAB EMIRATES
24.6.5 KUWAIT
24.6.6 OMAN
24.6.7 QATAR
24.6.8 SAUDI ARABIA
24.6.9 REST OF MEA
24.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL MYELOPROLIFERATIVE DISORDERS DRUGS MARKET, COMPANY PROFILE
25.1 PFIZER INC.
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 F. HOFFMANN-LA ROCHE LTD
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 NOVARTIS PHARMACEUTICALS CORPORATION
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 BRISTOL-MYERS SQUIBB
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 TAKEDA PHARMACEUITCAL COMPANY LIMITED
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 PHARACYCLICS LLC (AN ABBVIE COMPANY)
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 FRESENIUS KABI
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 HIKMA PHARMACEUTICALS PLC
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 TEVA PHARMACEUITCALS
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 MEDUNIK (SUBSIDIARY OF DUCHESNAY PHARMACEUTICAL GROUP INC.)
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 SUN PHARMACEUTICAL INDUSTRIES LTD.
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 SANOFI
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 CTI BIOPHARMA CORP.
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 PAR PHARMACEUITCAL
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 INCYTE
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












