Global Nanomedical Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
3.45 Billion
USD
7.54 Billion
2025
2033
USD
3.45 Billion
USD
7.54 Billion
2025
2033
| 2026 –2033 | |
| USD 3.45 Billion | |
| USD 7.54 Billion | |
|
|
|
|
Nanomedical Devices Market Size
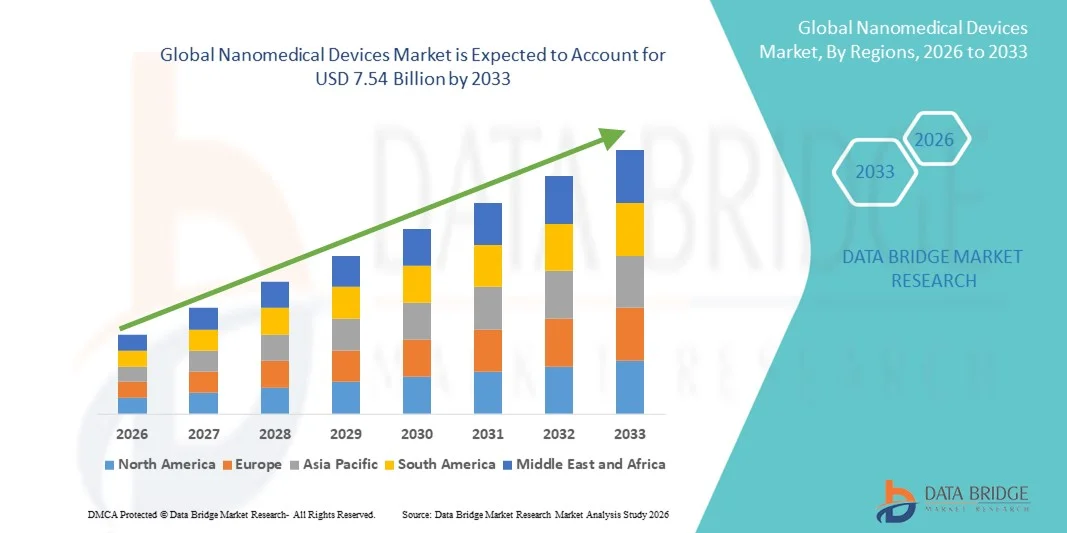
- The global nanomedical devices market size was valued at USD 3.45 billion in 2025 and is expected to reach USD 7.54 billion by 2033, at a CAGR of 10.82% during the forecast period
- The market growth is largely fueled by rapid advancements in nanotechnology, coupled with increasing integration of nanoscale solutions in medical diagnostics, drug delivery, and therapeutic applications, leading to improved treatment precision and patient outcomes across healthcare settings
- Furthermore, rising demand for minimally invasive, targeted, and personalized medical treatments, along with growing investments in nanomedicine research and development, is accelerating the uptake of nanomedical devices, thereby significantly boosting the overall growth of the Nanomedical Devices market
Nanomedical Devices Market Analysis
- Nanomedical devices, encompassing nanoscale diagnostic tools, drug-delivery systems, and implantable therapeutic technologies, are becoming increasingly critical in modern healthcare due to their ability to enable targeted therapy, early disease detection, and minimally invasive treatment approaches across clinical and research settings
- The escalating demand for nanomedical devices is primarily driven by the rising prevalence of chronic diseases, growing focus on precision and personalized medicine, and continuous advancements in nanotechnology that improve efficacy, safety, and patient outcomes
- North America dominated the nanomedical devices market with the largest revenue share of 42.3% in 2025, supported by strong R&D investments, early adoption of advanced medical technologies, a well-established healthcare infrastructure, and the presence of leading pharmaceutical, biotechnology, and medical device companies, particularly in the U.S.
- Asia-Pacific is expected to be the fastest-growing region in the nanomedical devices market during the forecast period, registering a robust CAGR of 10.8%, driven by expanding healthcare expenditure, rapid growth in biotechnology research, increasing government funding for nanomedicine, and a large patient pool across emerging economies
- The Disease Treatment and Diagnosis segment accounted for the largest market revenue share of around 46.8% in 2025, primarily due to the widespread use of nanomedical devices in oncology, cardiology, neurology, and metabolic disorder management
Report Scope and Nanomedical Devices Market Segmentation
|
Attributes |
Nanomedical Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
• Abbott (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Nanomedical Devices Market Trends
Rising Adoption of Nanotechnology-Based Devices for Precision Diagnosis and Therapy
- A significant and accelerating trend in the global nanomedical devices market is the increasing adoption of nanotechnology-enabled medical devices for targeted drug delivery, precision diagnostics, and minimally invasive therapeutic applications
- These devices operate at the nanoscale, allowing enhanced interaction with biological systems at the cellular and molecular levels
- For instance, nanoparticle-based diagnostic devices are increasingly being used in early cancer detection, enabling clinicians to identify tumors at very early stages through enhanced imaging contrast and biomarker sensitivity. Such applications are gaining traction across research hospitals and diagnostic laboratories worldwide
- Advances in nanomaterials such as liposomes, dendrimers, and polymeric nanoparticles are improving device performance by enhancing biocompatibility, stability, and controlled drug release
- The growing focus on personalized medicine is further driving the development of nanomedical devices that can deliver therapies precisely to affected tissues, reducing side effects and improving patient outcomes
- As regulatory frameworks gradually evolve to accommodate nanotechnology-based healthcare solutions, manufacturers are increasingly commercializing nanomedical devices for clinical use, reshaping the future of diagnostics and therapeutics globally
Nanomedical Devices Market Dynamics
Driver
Increasing Burden of Chronic Diseases and Demand for Advanced Medical Technologies
- The rising global prevalence of chronic diseases such as cancer, cardiovascular disorders, neurological conditions, and diabetes is a major driver fueling demand for nanomedical devices. These devices enable improved disease management through early diagnosis, targeted therapy, and real-time monitoring
- For instance, in 2024, several clinical research institutions in North America and Europe expanded the use of nano-enabled drug delivery devices to improve chemotherapy effectiveness while minimizing systemic toxicity, highlighting the growing clinical acceptance of nanomedical technologies
- Continuous growth in healthcare expenditure and medical research funding, particularly in developed regions such as North America and Europe, is supporting innovation and commercialization of nanomedical devices
- In addition, increasing investments in biotechnology and nanomedicine research across emerging economies in Asia-Pacific are accelerating product development and adoption
- The demand for minimally invasive and highly efficient treatment options is further strengthening the role of nanomedical devices in modern healthcare systems, driving sustained market growth
Restraint/Challenge
High Development Costs and Regulatory Complexity
- High research and development costs associated with nanomedical devices present a significant challenge to market growth. The complexity of designing, testing, and validating nanoscale devices requires advanced infrastructure, specialized expertise, and substantial financial investment
- For instance, many nanomedical devices face lengthy regulatory approval processes, as regulatory authorities require extensive safety, toxicity, and efficacy data due to the novel nature of nanomaterials used in medical applications
- Limited standardization in nanomedicine manufacturing and characterization methods can further slow commercialization and increase development timelines
- In addition, high production costs often result in premium-priced products, limiting accessibility in cost-sensitive healthcare markets, particularly in low- and middle-income countries
- Overcoming these challenges through streamlined regulatory pathways, international collaboration, and cost-efficient manufacturing techniques will be crucial for the long-term expansion of the global Nanomedical Devices market
Nanomedical Devices Market Scope
The market is segmented on the basis of type, communication protocol, unlocking mechanism, and application.
- By Type
On the basis of type, the Nanomedical Devices market is segmented into Implantable Biosensors, Implantable Cardioverter-Defibrillators (ICDs), and Others. The Implantable Biosensors segment dominated the largest market revenue share of approximately 41.6% in 2025, driven by their expanding role in continuous health monitoring and early disease detection. These devices enable real-time measurement of physiological parameters such as glucose levels, biomarkers, and metabolic indicators, supporting personalized and preventive healthcare. Rising prevalence of chronic diseases such as diabetes and cardiovascular disorders has significantly boosted demand for implantable biosensors. Technological advancements in nanomaterials, miniaturization, and biocompatibility have further improved device accuracy, lifespan, and patient comfort. In addition, increased integration with wireless data transmission and AI-based analytics enhances clinical decision-making. Growing adoption across hospitals and home-care settings reinforces their dominance. Strong investment from medical device manufacturers and favorable regulatory approvals also support sustained leadership of this segment.
The Implantable Cardioverter-Defibrillators (ICDs) segment is expected to witness the fastest CAGR of 18.9% from 2026 to 2033, driven by the rising global burden of cardiac arrhythmias and sudden cardiac arrest. Nanotechnology-enabled ICDs offer improved sensing accuracy, reduced device size, and longer battery life, enhancing patient safety and comfort. Increasing awareness of advanced cardiac care, especially in aging populations, further accelerates adoption. Technological integration with remote monitoring platforms allows continuous cardiac surveillance, reducing hospital visits. Expanding reimbursement coverage in developed economies and growing cardiac care infrastructure in emerging markets also contribute to rapid growth. These factors collectively position ICDs as the fastest-growing type segment.
- By Application
On the basis of application, the Nanomedical Devices market is segmented into Disease Treatment and Diagnosis, Drug Release Regulation, and Others. The Disease Treatment and Diagnosis segment accounted for the largest market revenue share of around 46.8% in 2025, primarily due to the widespread use of nanomedical devices in oncology, cardiology, neurology, and metabolic disorder management. Nanomedical technologies enable early detection, targeted therapy, and real-time monitoring, significantly improving treatment outcomes. The growing focus on precision medicine has accelerated the adoption of nanodevices for diagnostics and therapeutic interventions. Continuous innovation in nanoscale imaging agents and biosensors enhances diagnostic accuracy and treatment efficiency. Increasing healthcare expenditure and strong clinical validation further strengthen dominance. In addition, the rising prevalence of chronic and life-threatening diseases globally sustains high demand for disease-focused nanomedical applications.
The Drug Release Regulation segment is projected to register the fastest CAGR of 20.4% from 2026 to 2033, driven by advancements in controlled and targeted drug delivery systems. Nanomedical devices enable precise dosing, site-specific drug release, and reduced side effects, improving patient compliance and therapeutic efficacy. Growing demand for personalized drug delivery solutions, especially in cancer and autoimmune diseases, fuels rapid adoption. Ongoing R&D investments and successful clinical trials of nanocarrier-based release systems further accelerate growth. Pharmaceutical-medical device convergence and increasing approvals of nano-enabled therapies strongly position this segment for sustained expansion.
- By End-User
On the basis of end-user, the Nanomedical Devices market is segmented into Hospitals and Clinics, Research Institutes and Organizations, and Others. The Hospitals and Clinics segment held the largest market revenue share of approximately 52.3% in 2025, driven by high patient inflow, advanced medical infrastructure, and greater adoption of innovative treatment technologies. Hospitals serve as primary centers for implantation, monitoring, and management of nanomedical devices. Availability of skilled healthcare professionals and access to advanced diagnostic tools further supports widespread use. Rising investments in hospital modernization and increasing preference for minimally invasive procedures enhance demand. Moreover, favorable reimbursement policies and strong collaboration with device manufacturers reinforce hospital dominance. These factors collectively make hospitals and clinics the leading end-user segment.
The Research Institutes and Organizations segment is expected to witness the fastest CAGR of 17.6% from 2026 to 2033, fueled by increasing research funding and focus on nanotechnology-based medical innovation. Academic and research institutions play a critical role in developing next-generation nanomedical devices and validating clinical efficacy. Growing government and private investments in nanomedicine research accelerate adoption. Collaborations between universities, biotech firms, and healthcare providers further strengthen growth prospects. Rising emphasis on translational research and commercialization of nanomedical technologies positions this segment as the fastest-growing end-user category.
Nanomedical Devices Market Regional Analysis
- North America dominated the nanomedical devices market with the largest revenue share of 42.3% in 2025, supported by strong R&D investments, early adoption of advanced medical technologies, and a well-established healthcare infrastructure
- The region benefits from the presence of leading pharmaceutical, biotechnology, and medical device companies that actively invest in nanotechnology-based drug delivery systems, diagnostics, and therapeutic
- In addition, favorable regulatory frameworks, high healthcare expenditure, and strong collaboration between academic institutions and industry players continue to reinforce North America’s leadership position in the global nanomedical devices market
U.S. Nanomedical Devices Market Insight
The U.S. nanomedical devices market accounted for the largest revenue share within North America in 2025, driven by substantial federal and private funding for nanomedicine research and development. The country’s strong focus on precision medicine, targeted drug delivery, and advanced diagnostics is accelerating the adoption of nanomedical devices across oncology, cardiology, and infectious disease treatment. Moreover, the presence of major pharmaceutical companies, research universities, and innovation hubs significantly contributes to continuous product development and commercialization.
Europe Nanomedical Devices Market Insight
The Europe nanomedical devices market is projected to expand at a steady CAGR during the forecast period, primarily driven by growing investments in biomedical research and increasing adoption of advanced therapeutic technologies. Supportive government initiatives, rising prevalence of chronic diseases, and strong regulatory emphasis on patient safety are fostering market growth. Countries across Europe are increasingly focusing on nanotechnology-based solutions to improve treatment efficacy and reduce side effects.
U.K. Nanomedical Devices Market Insight
The U.K. nanomedical devices market is expected to grow at a notable CAGR during the forecast period, supported by strong academic research, government-backed innovation programs, and increasing clinical trials involving nanomedicine. The country’s emphasis on translational research and collaboration between universities and healthcare providers is promoting the adoption of nanomedical devices in diagnostics and targeted therapies.
Germany Nanomedical Devices Market Insight
The Germany nanomedical devices market is anticipated to witness considerable growth, driven by advanced healthcare infrastructure, high R&D spending, and a strong focus on medical innovation. Germany’s leadership in medical technology and engineering supports the development of high-quality nanomedical devices, particularly in drug delivery systems and imaging applications.
Asia-Pacific Nanomedical Devices Market Insight
The Asia-Pacific nanomedical devices market is expected to register the fastest CAGR of 10.8% during the forecast period, fueled by expanding healthcare expenditure, rapid growth in biotechnology research, and increasing government funding for nanomedicine. The region’s large patient population, rising burden of chronic diseases, and improving access to advanced healthcare technologies are significantly accelerating market expansion across emerging economies.
Japan Nanomedical Devices Market Insight
The Japan nanomedical devices market is witnessing steady growth, driven by advanced research capabilities, a strong pharmaceutical sector, and increasing demand for innovative medical solutions. Japan’s focus on precision medicine, aging population, and adoption of minimally invasive therapies is supporting the integration of nanomedical devices across multiple therapeutic areas.
China Nanomedical Devices Market Insight
The China nanomedical devices market held the largest revenue share in the Asia-Pacific region in 2025, attributed to rapid expansion of biotechnology research, increasing government investment in nanotechnology, and growing domestic manufacturing capabilities. Strong policy support for innovation, along with rising healthcare needs and a large patient pool, continues to drive the adoption of nanomedical devices across diagnostics and therapeutic applications.
Nanomedical Devices Market Share
The Nanomedical Devices industry is primarily led by well-established companies, including:
• Abbott (U.S.)
• Medtronic plc (Ireland)
• Boston Scientific Corporation (U.S.)
• Johnson & Johnson (U.S.)
• Siemens Healthineers (Germany)
• GE HealthCare (U.S.)
• Koninklijke Philips N.V. (Netherlands)
• Stryker Corporation (U.S.)
• Thermo Fisher Scientific (U.S.)
• Roche Holding AG (Switzerland)
• Nanospectra Biosciences (U.S.)
• Nanobiotix (France)
• Selective Diagnostics (U.S.)
• Becton, Dickinson and Company (U.S.)
• Oxford Instruments (U.K.)
• Bruker Corporation (U.S.)
• Merck KGaA (Germany)
• Agilent Technologies (U.S.)
• Celgene Corporation (U.S.)
• Tecomet Inc. (U.S.)
Latest Developments in Global Nanomedical Devices Market
- In December 2021, Exogenesis announced the completion of validation production and sterilization validation for its Nano Mesh hernia repair product, representing one of the early commercial validations of nanotechnology-based medical devices aimed at improved biocompatibility and clinical performance. This milestone underscores the transition of nanomedicine concepts into commercial medical applications with regulatory-aligned quality controls in place
- In April 2024, Satio and Nanowear entered into a strategic partnership to integrate Nanowear’s AI-based nanotechnology biomarker diagnostic platform with Satio’s at-home blood draw diagnostics and therapeutic patches, aiming to accelerate the adoption of remote, precision, and personalized healthcare solutions. This collaboration highlights the movement toward combining nanoscale sensing with real-world, patient-centric diagnostics and treatment devices
- In October 2024, UPM Biomedicals launched FibGel, the world’s first injectable nanocellulose hydrogel designed specifically for permanent implantable medical devices, offering a biocompatible, renewable alternative for drug delivery and tissue engineering applications. The introduction of FibGel marks an advancement in nanomaterial applications for implants and regenerative medicine products
- In August 2024, Evonik and KNAUER formed a strategic collaboration to scale and enhance manufacturing efficiency of lipid nanoparticle (LNP) formulations, a key enabling technology for mRNA and gene therapies, which are foundational nanomedical approaches for targeted therapeutics. Their joint efforts aim to optimize pre-clinical development and production processes for nanomedicine components, accelerating delivery timelines for novel therapeutic candidates
- In November 2024, Ardena, a contract development and manufacturing organization, secured full Good Manufacturing Practice (GMP) certification for its expanded nanomedicine manufacturing facility in Oss, Netherlands, allowing the production of nanomedicines under rigorous regulatory standards and enhancing its role in supporting next-generation drug development. This certification strengthens Ardena’s capacity to deliver high-quality nanotechnology-based solutions
- In April 2025, the global forecast for Nanorobots for Drug Delivery estimated market expansion with exogenous nanorobots leading next-generation chemotherapy and radiotherapy solutions, reflecting growing commercial and clinical interest in precision nanomedical actuation platforms. Nanorobots represent a frontier in nanomedical devices capable of navigating the body and delivering therapies directly to targeted tissues
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.