Global Needle Free Iv Connectors Market
Market Size in USD Million
CAGR :
% 
 USD
995.54 Million
USD
1,857.74 Million
2024
2032
USD
995.54 Million
USD
1,857.74 Million
2024
2032
| 2025 –2032 | |
| USD 995.54 Million | |
| USD 1,857.74 Million | |
|
|
|
|
Needle Free Iv Connectors Market Size
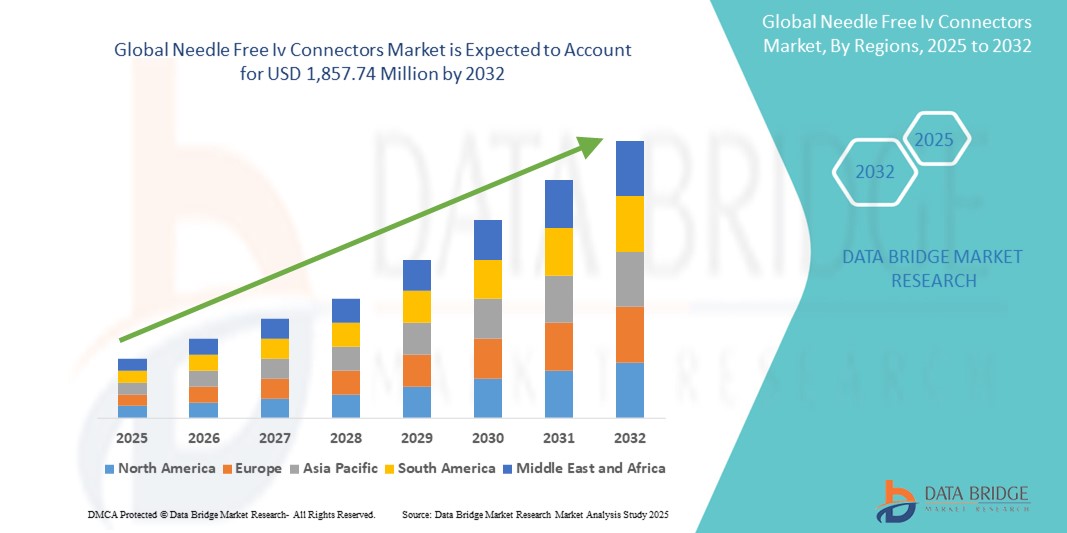
- The global needle free IV connectors market size was valued at USD 995.54 million in 2024 and is expected to reach USD 1,857.74 million by 2032, at a CAGR of 8.11% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within intravenous (IV) therapy equipment and infection prevention technologies, leading to increased digitalization and safety in both hospital and homecare settings
- Furthermore, rising healthcare professional demand for secure, user-friendly, and contamination-free IV access solutions is establishing needle-free IV connectors as the preferred option for reducing bloodstream infection risks. These converging factors are accelerating the uptake of needle-free IV connector solutions, thereby significantly boosting the industry's growth
Needle Free Iv Connectors Market Analysis
- Needle-free IV connectors, designed to reduce the risk of needlestick injuries and prevent catheter-related bloodstream infections, are becoming essential components in modern intravenous therapy practices across hospitals, clinics, and homecare settings due to their safety, ease of use, and infection control benefits
- The escalating demand for needle-free IV connectors is primarily fueled by the increasing prevalence of chronic diseases, rising hospital-acquired infection (HAI) rates, and growing awareness around safe infusion practices among healthcare professionals
- North America dominated the needle-free IV connectors market with the largest revenue share of 42.8% in 2024, driven by advanced healthcare infrastructure, high patient safety standards, and a strong presence of key industry players. The U.S. is witnessing significant adoption of needle-free IV connectors across both inpatient and outpatient care, supported by stringent regulatory policies and continuous product innovation
- Asia-Pacific is expected to be the fastest-growing region in the needle-free IV connectors market during the forecast period, projected to grow at a CAGR of 9.6% from 2025 to 2032, due to increasing healthcare investments, rising incidence of infectious diseases, and rapid expansion of hospital infrastructure in countries such as India, China, and Indonesia
- The straight channel segment dominated the needle-free IV connectors market with the largest market revenue share of 38.6% in 2024, driven by its simplicity, ease of use, and widespread acceptance in hospitals and clinics. Straight connectors are commonly used in intravenous therapy due to their efficient design, which reduces the risk of contamination and provides reliable fluid transfer
Report Scope and Needle Free Iv Connectors Market Segmentation
|
Attributes |
Needle Free IV Connectors Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Needle Free Iv Connectors Market Trends
“Increased Emphasis on Infection Control and Patient Safety”
- A significant and accelerating trend in the global needle free IV connectors market is the growing emphasis on infection prevention and patient safety within healthcare settings. The shift from traditional needle-based systems to closed, needle-free alternatives is reshaping clinical practices globally
- For instance, leading healthcare institutions have reported a measurable reduction in catheter-related bloodstream infections (CRBSIs) following the adoption of needle-free connectors, highlighting their effectiveness in improving patient outcomes
- These connectors reduce the risk of needlestick injuries for healthcare workers and minimize microbial ingress, a key factor in hospital-acquired infections. Advanced connector designs are now incorporating anti-reflux mechanisms, positive displacement, and closed systems to enhance fluid integrity and reduce contamination
- Furthermore, global regulatory guidelines—including those from the CDC, WHO, and FDA—strongly support the adoption of needle-free IV systems, accelerating their use in both inpatient and outpatient care settings
- As healthcare providers increasingly shift towards value-based care and infection prevention becomes a top priority, the demand for safe, user-friendly, and effective IV access devices continue to rise
- This trend toward safer infusion practices is fundamentally transforming the way intravenous therapies are administered across hospitals, ambulatory centers, and homecare environments. Companies such as ICU Medical, Becton Dickinson and Company, and Baxter are actively developing next-generation needle-free connectors to meet evolving clinical needs and regulatory standards
- The growing demand for needle-free IV connectors, supported by strong evidence of their safety benefits and cost-effectiveness, is driving rapid market expansion globally, especially in developed healthcare systems and increasingly in emerging economies.
Needle Free Iv Connectors Market Dynamics
Driver
“Growing Need Due to Rising Hospital-Acquired Infections and Demand for Safe Infusion Systems”
- The increasing prevalence of hospital-acquired infections (HAIs) and the need to reduce catheter-related bloodstream infections (CRBSIs) are significant drivers fueling the demand for needle-free IV connectors. These connectors minimize the risk of contamination, offering a safer alternative to traditional systems
- For instance, the Centers for Disease Control and Prevention (CDC) emphasizes the importance of closed systems such as needle-free connectors in infection control protocols, especially in ICUs and oncology wards
- As hospitals and outpatient facilities increasingly shift towards closed IV systems for safer medication administration, needle-free connectors are becoming a critical part of intravenous therapy
- Furthermore, the rise in chronic disease prevalence, including cancer and diabetes, which require frequent IV access, is propelling the adoption of these connectors across home care and ambulatory surgical centers
- The need for user-friendly, efficient, and compatible infusion solutions—especially in home settings—has driven the development of more ergonomic, secure, and multi-channel designs within needle-free IV connector systems
- The expansion of minimally invasive procedures and value-based healthcare models is also encouraging healthcare providers to adopt cost-effective solutions that improve patient outcomes and reduce hospital stays
- As medical device regulations become stricter in regions such as the U.S. and Europe, manufacturers are compelled to offer high-quality, sterile, and compliant needle-free connectors, thereby enhancing product quality and trust in these devices
Restraint/Challenge
“Complexity in Design and Concerns Regarding Device-Related Infections”
- One of the key restraints limiting the growth of the needle-free IV connectors market is the variation in connector design, which may inadvertently contribute to microbial ingress or backflow—posing infection risks
- Device-related infections can occur if the connectors are not disinfected properly or if incompatible devices are used, leading to complications and increased healthcare costs
- For instance, the FDA has raised concerns about certain designs, especially those with negative pressure mechanisms, which may have a higher association with bloodstream infections when not used with proper flushing techniques
- This challenge has pushed healthcare providers to standardize IV connector use, which can restrict product diversity and limit new entrants in the market
- In addition, the high cost of advanced connectors, particularly those with antimicrobial coatings or multi-channel access points, may deter adoption in resource-limited settings, especially in developing nations
- Training and education gaps regarding the proper usage, cleaning, and maintenance of these devices can also compromise their effectiveness, hindering full market potential
- Overcoming these hurdles will require collaborative efforts between manufacturers, healthcare providers, and regulatory authorities to develop cost-effective, infection-resistant, and user-friendly designs that promote safety and efficacy in intravenous therapy
Needle Free Iv Connectors Market Scope
The market is segmented on the basis of design type, mechanism, dwell time, and end-user.
• By Design Type
On the basis of design type, the needle free IV connectors market is segmented into straight channel, T-channel, Y-channel, and multi-channel. The straight channel segment dominated the largest market revenue share of 38.6% in 2024, driven by its simplicity, ease of use, and widespread acceptance in hospitals and clinics. Straight connectors are commonly used in intravenous therapy due to their efficient design, which reduces the risk of contamination and provides reliable fluid transfer.
The multi-channel segment is anticipated to witness the fastest growth rate of 23.4% from 2025 to 2032, due to its ability to support multiple fluid pathways simultaneously. These connectors offer flexibility in drug administration and are increasingly adopted in complex patient care settings such as ICUs and oncology units.
• By Mechanism
On the basis of mechanism, the needle free IV connectors market is segmented into positive, negative, and neutral. The neutral mechanism segment held the largest market revenue share of 41.2% in 2024, owing to its ability to minimize the risk of catheter-related bloodstream infections (CRBSIs). These connectors are preferred for their balanced flow and safety features, reducing complications associated with reflux.
The positive mechanism segment is expected to witness the fastest CAGR of 22.1% from 2025 to 2032, attributed to its one-way valve system that prevents backflow of blood into the catheter, thereby decreasing infection risk and improving patient outcomes.
• By Dwell Time
On the basis of dwell time, the needle free IV connectors market is segmented into seven-day and other than seven-day. The seven-day segment accounted for the largest market share of 67.5% in 2024, driven by its compatibility with standard infusion protocols and reduced frequency of device changes, which lowers patient discomfort and healthcare costs.
The other than seven-day segment is projected to grow at a fastest CAGR of 20.3% from 2025 to 2032, due to emerging products tailored for short-term procedures or high-risk patients who require frequent line access changes.
• By End-User
On the basis of end-user, the needle free IV connectors market is segmented into hospitals, ambulatory surgical centers, and others. The hospitals segment captured the largest revenue share of 65.9% in 2024, fueled by the high volume of inpatient admissions, growing prevalence of chronic illnesses, and robust demand for infection prevention devices.
The ambulatory surgical centers segment is expected to grow at the fastest CAGR of 21.4% from 2025 to 2032, as outpatient care gains traction and these centers increasingly adopt advanced needle-free technologies to enhance safety, streamline operations, and comply with stringent infection control standards.
Needle Free Iv Connectors Market Regional Analysis
- North America dominated the needle free IV connectors market with the largest revenue share of 42.8% in 2024, driven by advanced healthcare infrastructure, growing prevalence of chronic conditions, and strong emphasis on reducing catheter-related bloodstream infections (CRBSIs) through closed-system IV access technologies
- Hospitals and ambulatory surgical centers across the region increasingly adopt needle-free connectors to improve patient safety and minimize needlestick injuries. The region’s robust regulatory support and healthcare digitization further boost product demand
- The presence of key market players, favorable reimbursement policies, and a shift towards home-based infusion therapies continue to solidify North America’s leadership in this space
U.S. Needle Free IV Connectors Market Insight
The U.S. needle free IV connectors market captured the largest revenue share of 81.05% within North America in 2024, fueled by high infection prevention standards set by the CDC and FDA, rising hospital-acquired infection (HAI) awareness, and adoption of needleless systems across both inpatient and outpatient settings. The market benefits from widespread implementation of home infusion therapies, aging patient demographics, and strong demand for technologically advanced IV connectors.
Europe Needle Free IV Connectors Market Insight
The Europe needle free IV connectors market is projected to expand at a CAGR of 7.2% during 2025–2032, driven by supportive healthcare infrastructure, strict infection prevention regulations, and growing demand for safe IV therapy solutions. Key countries such as Germany, the U.K., and France are increasingly emphasizing closed-system devices in hospitals to mitigate bloodstream infection risks.
U.K. Needle Free IV Connectors Market Insight
The U.K. needle free IV connectors market accounted for 13.5% of the Europe Needle Free IV Connectors market in 2024, and is anticipated to grow at a notable CAGR of 6.8% during the forecast period. This growth is driven by strong NHS-backed initiatives promoting HBV screening, broader infection prevention programs, and increased focus on patient safety in infusion practices.
Germany Needle Free IV Connectors Market Insight
The Germany needle free IV connectors market contributed approximately 18% of Europe’s revenue share in 2024, supported by a technologically advanced medical ecosystem, preference for eco-friendly and disposable medical products, and increased investment in ICU and emergency care infrastructure. The country’s push toward digitized and integrated care systems is further accelerating the demand for needleless IV connectors.
Asia-Pacific Needle Free IV Connectors Market Insight
The Asia-Pacific needle free IV connectors market is poised to grow at the fastest CAGR of 9.6% from 2025 to 2032, led by increasing access to healthcare, rapid hospital infrastructure expansion, and growing awareness of infection control measures. Countries such as China, Japan, and India are investing heavily in safe infusion technologies, making the region a vital hub for market expansion and manufacturing.
Japan Needle Free IV Connectors Market Insight
The Japan needle free IV connectors market accounted for approximately 12% of the Asia-Pacific market revenue in 2024, driven by its aging population, advanced healthcare infrastructure, and proactive infection prevention practices. Hospitals and homecare providers are increasingly adopting needle-free systems due to their safety, ease of use, and compatibility with modern infusion equipment.
China Needle Free IV Connectors Market Insight
The China needle free IV connectors market captured 45% of the Asia-Pacific revenue share in 2024, making it the largest contributor in the region. This dominance stems from its rapidly growing healthcare sector, expansion of tertiary hospitals, favorable government healthcare reforms, and widespread adoption of closed-system IV devices to reduce CRBSIs. In addition, China's position as a manufacturing powerhouse supports affordable and scalable production of these devices.
Needle Free Iv Connectors Market Share
The Needle Free Iv Connectors industry is primarily led by well-established companies, including:
- BD (U.S.)
- Ascor SA (Poland)
- Smiths Group plc (U.K.)
- B. Braun SE (Germany)
- AngioDynamics (U.S.)
- Terumo Corporation (Japan)
- RyMed Technologies, LLC (U.S.)
- Baxter (U.S.)
- Nexus Medical (U.S.)
- Vygon (France)
- CU Medical Germany GmbH (South Korea)
Latest Developments in Global Needle Free Iv Connectors Market
- In November 2023, BD introduced the PIVOT Pro Needle-free Blood Collection Device, designed to work seamlessly with both integrated and long peripheral IV catheters. This innovative product aligns with BD's vision of achieving a "One-Stick Hospital Stay," aiming to enhance patient comfort and streamline blood collection procedures. The launch highlights BD's commitment to improving healthcare efficiency through advanced medical technologies
- In September 2023, PharmaJet, known for its precision delivery systems, announced encouraging results from Scancell's Phase 2 clinical trial targeting patients with unresectable advanced melanoma. The trial utilized the PharmaJet Stratis System for needle-free injection, which has emerged as a favored method among patients. This positive data reinforces the effectiveness of needle-free technology in enhancing patient experience and treatment outcomes
- In September 2023, the Indian government launched GEMCOVAC-OM, a COVID-19 booster vaccine specifically designed with mRNA targeting the Omicron variant. Administered intradermally through a needle-free injector, this thermostable vaccine demonstrated enhanced immune responses in study participants. The innovative delivery method contributes to its effectiveness, marking a significant advancement in vaccination strategies
- In August 2023, Pulse Needle Free Systems launched the first-ever line of disposable needle-free vaccination equipment for animals. Priced comparably to traditional syringes and needles, these innovative devices offer significant health and food safety benefits for pig farmers. This development marks a notable advancement in veterinary vaccination methods, enhancing efficiency and reducing the risk of infection
- In June 2023, PharmaJet partnered with Zydus Lifesciences to successfully administer the world’s first plasmid DNA COVID-19 vaccine utilizing its Tropis system. This innovative delivery method has demonstrated enhanced immune responses and improved clinical effectiveness. This collaboration marks a significant milestone in vaccine technology, showcasing the potential of needle-free delivery systems in combating infectious diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1. INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL NEEDLE FREE IV CONNECTORS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2. MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL NEEDLE FREE IV CONNECTORS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VALUE AND VOLUME
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL NEEDLE FREE IV CONNECTORS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3. MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4. EXECUTIVE SUMMARY
5. PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6. INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7. INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8. COST ANALYSIS BREAKDOWN
9. TECHNONLOGY ROADMAP
10. INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11. REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12. REIMBURSEMENT FRAMEWORK
13. OPPUTUNITY MAP ANALYSIS
14. VALUE CHAIN ANALYSIS
15. HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.10 ECONOMIC DEVELOPMENT
16. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, BY PRODUCT
16.1 OVERVIEW
16.2 STRAIGHT CHANNEL
16.2.1 BY TUBE LUMEN
16.2.1.1. SINGLE
16.2.1.2. DOUBLE
16.2.1.3. TRIPLE
16.2.2 BY TUBE LENGTH
16.2.2.1. 10 CM
16.2.2.2. 15 CM
16.2.2.3. 30 CM
16.2.2.4. OTHERS
16.2.3 BY BORE SIZE
16.2.3.1. SMALL BORE
16.2.3.2. LARGE BORE
16.2.4 BY MATERIAL
16.2.4.1. SILICONE
16.2.4.2. PLASTIC
16.2.4.3. POLYVINYL CHLORIDE (PVC)
16.2.4.4. POLYCARBONATE OR COPOLYESTER
16.2.4.5. OTHERS
16.2.5 BY HYGEINE
16.2.5.1. STERILE
16.2.5.2. NON-STERILE
16.2.6 BY COLOUR
16.2.6.1. YELLOW
16.2.6.2. GREEN
16.2.6.3. RED
16.2.6.4. OTHERS
16.2.7 OTHERS
16.3 Y CHANNEL
16.3.1 BY TUBE LUMEN
16.3.1.1. SINGLE
16.3.1.2. DOUBLE
16.3.1.3. TRIPLE
16.3.2 BY TUBE LENGTH
16.3.2.1. 10 CM
16.3.2.2. 15 CM
16.3.2.3. 30 CM
16.3.2.4. OTHERS
16.3.3 BY BORE SIZE
16.3.3.1. SMALL BORE
16.3.3.2. LARGE BORE
16.3.4 BY MATERIAL
16.3.4.1. SILICONE
16.3.4.2. PLASTIC
16.3.4.3. POLYVINYL CHLORIDE (PVC)
16.3.4.4. POLYCARBONATE OR COPOLYESTER
16.3.4.5. OTHERS
16.3.5 BY HYGEINE
16.3.5.1. STERILE
16.3.5.2. NON-STERILE
16.3.6 BY COLOUR
16.3.6.1. YELLOW
16.3.6.2. GREEN
16.3.6.3. RED
16.3.6.4. OTHERS
16.3.7 OTHERS
16.4 T CHANNEL
16.4.1 BY TUBE LUMEN
16.4.1.1. SINGLE
16.4.1.2. DOUBLE
16.4.1.3. TRIPLE
16.4.2 BY TUBE LENGTH
16.4.2.1. 10 CM
16.4.2.2. 15 CM
16.4.2.3. 30 CM
16.4.2.4. OTHERS
16.4.3 BY BORE SIZE
16.4.3.1. SMALL BORE
16.4.3.2. LARGE BORE
16.4.4 BY MATERIAL
16.4.4.1. SILICONE
16.4.4.2. PLASTIC
16.4.4.3. POLYVINYL CHLORIDE (PVC)
16.4.4.4. POLYCARBONATE OR COPOLYESTER
16.4.4.5. OTHERS
16.4.5 BY HYGEINE
16.4.5.1. STERILE
16.4.5.2. NON-STERILE
16.4.6 BY COLOUR
16.4.6.1. YELLOW
16.4.6.2. GREEN
16.4.6.3. RED
16.4.6.4. OTHERS
16.4.7 OTHERS
16.5 MULTI CHANNEL
16.5.1 BY TUBE LUMEN
16.5.1.1. SINGLE
16.5.1.2. DOUBLE
16.5.1.3. TRIPLE
16.5.2 BY TUBE LENGTH
16.5.2.1. 10 CM
16.5.2.2. 15 CM
16.5.2.3. 30 CM
16.5.2.4. OTHERS
16.5.3 BY BORE SIZE
16.5.3.1. SMALL BORE
16.5.3.2. LARGE BORE
16.5.4 BY MATERIAL
16.5.4.1. SILICONE
16.5.4.2. PLASTIC
16.5.4.3. POLYVINYL CHLORIDE (PVC)
16.5.4.4. POLYCARBONATE OR COPOLYESTER
16.5.4.5. OTHERS
16.5.5 BY HYGEINE
16.5.5.1. STERILE
16.5.5.2. NON-STERILE
16.5.6 BY COLOUR
16.5.6.1. YELLOW
16.5.6.2. GREEN
16.5.6.3. RED
16.5.6.4. OTHERS
16.5.7 OTHERS
17. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, BY TYPE
17.1 OVERVIEW
17.2 POSITIVE FLUID DISPLACEMENT
17.3 NEGATIVE FLUID DISPLACEMENT
17.4 NEUTRAL FLUID DISPLACEMENT
18. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, BY MATERIAL
18.1 OVERVIEW
18.2 SILICONE
18.3 PLASTIC
18.4 POLYVINYL CHLORIDE (PVC)
18.5 POLYCARBONATE OR COPOLYESTER
18.6 OTHERS
19. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, BY HYGEINE
19.1 OVERVIEW
19.2 STERILE
19.3 NON-STERILE
20. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, BY PACKAGING
20.1 OVERVIEW
20.2 50/BOX
20.3 100/BOX
20.4 OTHERS
21. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, BY APPLICATION
21.1 OVERVIEW
21.2 UROLOGY
21.3 CARDIOLOGY
21.4 BLOOD PROCESSING
21.5 OTHERS
22. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, BY END USER
22.1 OVERVIEW
22.2 HOSPITALS
22.2.1 BY TYPE
22.2.1.1. PUBLIC
22.2.1.2. PRIVATE
22.2.2 BY LEVEL
22.2.2.1. TIER 1
22.2.2.2. TIER 2
22.2.2.3. TIER 3
22.3 SPECIALTY CLINICS
22.3.1 PUBLIC
22.3.2 PRIVATE
22.4 HOME CARE SETTINGS
22.5 AMBULATORY SURGICAL CENTRE
22.6 ACADEMIC AND RESEARCH INSTITUTES
22.7 OTHERS
23. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, BY DISTRIBUTION CHANNEL
23.1 OVERVIEW
23.2 DIRECT TENDER
23.3 RETAIL SALES
23.3.1 OFFLINE
23.3.1.1. HOSPITAL PAHRMACY
23.3.1.2. DRUGS STORES
23.3.1.3. OTHERS
23.3.2 ONLINE
23.3.2.1. E-STORES
23.3.2.2. COMPANY WEBSITE
23.3.2.3. OTHERS
23.4 OTHERS
24. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, BY GEOGRAPHY
GLOBAL NEEDLE FREE IV CONNECTORS MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.1 NORTH AMERICA
24.1.1 U.S.
24.1.2 CANADA
24.1.3 MEXICO
24.2 EUROPE
24.2.1 GERMANY
24.2.2 FRANCE
24.2.3 U.K.
24.2.4 ITALY
24.2.5 SPAIN
24.2.6 RUSSIA
24.2.7 TURKEY
24.2.8 BELGIUM
24.2.9 DENMARK
24.2.10 NETHERLANDS
24.2.11 SWITZERLAND
24.2.12 SWEDEN
24.2.13 POLAND
24.2.14 NORWAY
24.2.15 FINLAND
24.2.16 REST OF EUROPE
24.3 ASIA-PACIFIC
24.3.1 JAPAN
24.3.2 CHINA
24.3.3 SOUTH KOREA
24.3.4 INDIA
24.3.5 AUSTRALIA
24.3.6 NEW ZEALAND
24.3.7 SINGAPORE
24.3.8 THAILAND
24.3.9 MALAYSIA
24.3.10 VIETNAM
24.3.11 TAIWAN
24.3.12 INDONESIA
24.3.13 PHILIPPINES
24.3.14 REST OF ASIA-PACIFIC
24.4 SOUTH AMERICA
24.4.1 BRAZIL
24.4.2 ARGENTINA
24.4.3 REST OF SOUTH AMERICA
24.5 MIDDLE EAST AND AFRICA
24.5.1 SOUTH AFRICA
24.5.2 SAUDI ARABIA
24.5.3 BAHRAIN
24.5.4 UAE
24.5.5 KUWAIT
24.5.6 OMAN
24.5.7 QATAR
24.5.8 EGYPT
24.5.9 ISRAEL
24.5.10 REST OF MIDDLE EAST AND AFRICA
24.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, SWOT AND DBMR ANALYSIS
26. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, COMPANY LANDSCAPE
26.1 COMPANY SHARE ANALYSIS: GLOBAL
26.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
26.3 COMPANY SHARE ANALYSIS: EUROPE
26.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
26.5 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
26.6 MERGERS & ACQUISITIONS
26.7 NEW PRODUCT DEVELOPMENT & APPROVALS
26.8 EXPANSIONS
26.9 REGULATORY CHANGES
26.10 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
27. GLOBAL NEEDLE FREE IV CONNECTORS MARKET, COMPANY PROFILE
27.1 BD
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPMENTS
27.2 ROMSONS
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPMENTS
27.3 NP MEDICAL
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPMENTS
27.4 NORDSON CORPORATION
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPMENTS
27.5 VYGON GROUP
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPMENTS
27.6 BAIHE MEDICAL EUROPE
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPMENTS
27.7 ADVACARE PHARMA
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPMENTS
27.8 LEPU MEDICAL TECHNOLOGY(BEIJING)CO.,LTD.
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPMENTS
27.9 ICU MEDICAL , INC.
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPMENTS
27.10 POLYMEDICURE
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPMENTS
27.11 LARS MEDICARE PVT. LTD
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPMENTS
27.12 HANGZHOU FUSHAN MEDICAL APPLIANCES CO., LTD.
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPMENTS
27.13 BAXTER
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPMENTS
27.14 NEXUS MEDICAL
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPMENTS
27.15 MAIS INDIA
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPMENTS
27.16 SPARK LIFESCIENCES
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPMENTS
27.17 ASSET MEDICAL
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPMENTS
27.18 TERUMO CORPORATION
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPMENTS
27.19 RYMED TECHNOLOGIES, LLC
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPMENTS
27.20 INDOSURGICALS PRIVATE LIMITED
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPMENTS
27.21 WEIGAO MEIDCAL INTERNATIONAL CO., LTD
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.21.5 RECENT DEVELOPMENTS
27.22 HUBIOMED INC.
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.22.5 RECENT DEVELOPMENTS
27.23 KINDLY(KDL) MEDITECH
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.23.5 RECENT DEVELOPMENTS
27.24 SHANGHAI INT MEDICAL INSTRUMENTS CO., LTD.
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.24.5 RECENT DEVELOPMENTS
27.25 KAPSAM HEALTH PRODUCTS
27.25.1 COMPANY OVERVIEW
27.25.2 REVENUE ANALYSIS
27.25.3 GEOGRAPHIC PRESENCE
27.25.4 PRODUCT PORTFOLIO
27.25.5 RECENT DEVELOPMENTS
27.26 HALKEY-ROBERTS CORPORATION
27.26.1 COMPANY OVERVIEW
27.26.2 REVENUE ANALYSIS
27.26.3 GEOGRAPHIC PRESENCE
27.26.4 PRODUCT PORTFOLIO
27.26.5 RECENT DEVELOPMENTS
27.27 EIB CO., LTD
27.27.1 COMPANY OVERVIEW
27.27.2 REVENUE ANALYSIS
27.27.3 GEOGRAPHIC PRESENCE
27.27.4 PRODUCT PORTFOLIO
27.27.5 RECENT DEVELOPMENTS
27.28 MEDIPLUS LTD.
27.28.1 COMPANY OVERVIEW
27.28.2 REVENUE ANALYSIS
27.28.3 GEOGRAPHIC PRESENCE
27.28.4 PRODUCT PORTFOLIO
27.28.5 RECENT DEVELOPMENTS
28. RELATED REPORT
29. CONCLUSION
30. QUESTIONNAIRE
31. ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.