Global Neonatal Jaundice Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
196.20 Million
USD
328.49 Million
2024
2032
USD
196.20 Million
USD
328.49 Million
2024
2032
| 2025 –2032 | |
| USD 196.20 Million | |
| USD 328.49 Million | |
|
|
|
|
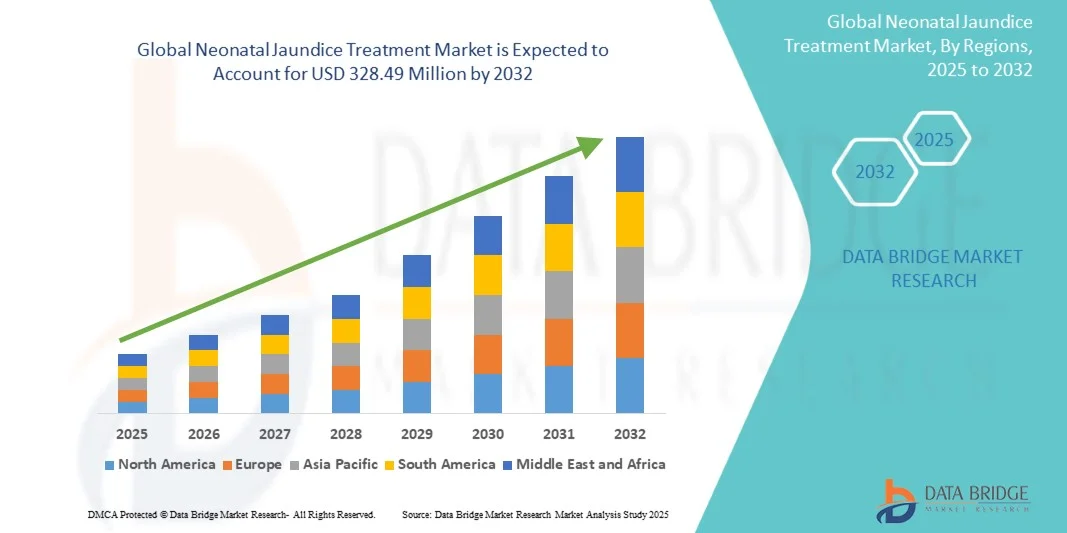
Neonatal Jaundice Treatment Market Size
- The global neonatal jaundice treatment market size was valued at USD 196.20 Million in 2024 and is expected to reach USD 328.49 Million by 2032, at a CAGR of 6.65% during the forecast period
- The market growth is largely fueled by the increasing prevalence of neonatal jaundice and rising awareness among healthcare providers and parents regarding timely diagnosis and effective treatment options
- Furthermore, growing demand for safe, efficient, and easy-to-administer therapies for newborns is driving the adoption of neonatal jaundice treatment solutions. These converging factors are accelerating the uptake of phototherapy devices, medications, and supportive treatments, thereby significantly boosting the industry’s growth
Neonatal Jaundice Treatment Market Analysis
- Neonatal Jaundice Treatments, offering medical therapies and phototherapy solutions, are increasingly vital for managing bilirubin levels in newborns, reducing the risk of severe complications, and improving neonatal outcomes in both hospital and home-care settings
- The escalating demand for neonatal jaundice treatments is primarily fueled by rising awareness of early detection, growing prevalence of neonatal hyperbilirubinemia, and advancements in treatment modalities, including LED phototherapy and pharmacological interventions
- North America dominated the neonatal jaundice treatment market with the largest revenue share of 41.5% in 2024, characterized by advanced healthcare infrastructure, high awareness of neonatal care, and strong presence of key pharmaceutical and medical device companies. The U.S. experienced substantial growth in Neonatal Jaundice Treatments, particularly in hospital-based and outpatient care settings, driven by innovations from both established companies and specialized neonatal care startups focusing on effective and safer therapies
- Asia-Pacific is expected to be the fastest-growing region in the neonatal jaundice treatment market during the forecast period, with a projected CAGR of 23.5%, due to increasing urbanization, rising healthcare expenditure, expanding neonatal care facilities, and growing awareness of early treatment benefits in countries such as China, India, and Japan
- The Neonatal Intensive Care Units segment dominated the neonatal jaundice treatment market with a market revenue share of 50.2% in 2024, due to higher incidence of jaundice in premature and high-risk infants. NICUs have specialized equipment and trained personnel to administer treatments
Report Scope and Neonatal Jaundice Treatment Market Segmentation
|
Attributes |
Neonatal Jaundice Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Neonatal Jaundice Treatment Market Trends
“Advancements and Adoption Trends in Neonatal Jaundice Treatment”
- A significant and accelerating trend in the global neonatal jaundice treatment market is the growing use of advanced phototherapy devices, innovative pharmacological therapies, and home-based neonatal care solutions that enhance clinical outcomes
- For instance, high-intensity LED phototherapy systems are increasingly being implemented in hospitals and neonatal intensive care units (NICUs), offering more effective and safer treatment compared to conventional phototherapy lamps
- The trend towards combination therapies, including pharmacological interventions alongside phototherapy, is gaining traction to reduce treatment duration and prevent complications such as kernicterus
- Portable and low-cost phototherapy units are also being introduced, enabling treatment in home-care settings and remote areas where hospital access is limited
- Enhanced awareness campaigns among healthcare professionals and parents are driving early diagnosis and timely intervention, contributing to improved treatment outcomes
- Growing emphasis on evidence-based protocols and standardized treatment guidelines is further shaping clinical practices and influencing product adoption
Neonatal Jaundice Treatment Market Dynamics
Driver
“Rising Awareness and Expansion of Advanced Therapies”
- The rising global incidence of neonatal jaundice is significantly driving the demand for effective treatment options, including phototherapy and pharmacological interventions
- Increasing awareness among parents and healthcare providers about the severe complications of untreated jaundice, such as kernicterus, is encouraging timely adoption of treatment
- For Instance: In 2024, the introduction of advanced LED phototherapy devices in multiple hospitals across India resulted in faster bilirubin reduction and improved neonatal recovery rates, illustrating the tangible benefits of technology adoption in this market.
- Expansion of neonatal healthcare facilities, NICUs, and specialized clinics in both developed and emerging regions is improving access to advanced neonatal care
- Introduction of combination therapies and safer pharmacological interventions is enhancing treatment effectiveness and patient outcomes
- Evidence-based treatment protocols and clinical guidelines are promoting standardization of care, ensuring that hospitals and clinics adopt effective therapies with confidence
- Shortened hospital stays and availability of home-based phototherapy solutions are increasing convenience for parents and supporting wider adoption
- Government initiatives, such as maternal and child health programs, are facilitating access to neonatal care and encouraging hospitals to adopt modern treatment solutions
- Increasing research and development activities focusing on advanced phototherapy devices, including LED-based systems and bilirubin monitoring technology, are driving innovation and improving efficiency
- Strategic partnerships between hospitals, medical device manufacturers, and NGOs are enhancing awareness, distribution networks, and treatment accessibility in underserved regions
- Rising disposable incomes and higher healthcare spending in emerging economies are enabling parents to access modern treatment solutions
Restraint/Challenge
“Safety Concerns, Cost Barriers, and Regulatory Limitations”
- Regulatory restrictions and strict safety requirements in various regions can slow the adoption of new treatment solutions. Hospitals and clinics must comply with local regulations, which may involve lengthy approval processes
- For Instance: In 2023, a temporary recall of a neonatal phototherapy device in Europe due to overheating issues highlighted the importance of stringent safety monitoring and regulatory compliance, demonstrating how safety concerns can impact market adoption
- Competition from alternative therapies, such as traditional sunlight exposure, older pharmacological options, and locally-preferred practices, can limit market penetration in some regions
- The need for robust clinical evidence, post-marketing surveillance, and ongoing research to ensure long-term safety can increase operational costs for manufacturers and healthcare providers
- High cost of advanced phototherapy equipment or combination drug therapies can be a significant barrier, particularly in developing economies or for price-sensitive healthcare providers
- Lack of trained healthcare personnel in some regions can restrict the adoption of advanced neonatal treatment technologies
- Supply chain limitations and occasional device recalls can disrupt availability and impact market confidence
- While generic formulations improve affordability, premium or branded therapies often come at higher costs, which can affect adoption among economically constrained segments
- Collaboration between regulatory authorities, hospitals, and manufacturers is essential to maintain consistent availability, ensure safety, and build trust in modern therapies
- Integration of telemedicine and remote monitoring in neonatal care is still limited in certain regions due to infrastructure challenges, slowing adoption rates
Neonatal Jaundice Treatment Market Scope
The market is segmented on the basis of type, end-user, treatment mechanism, and application.
• By Type
On the basis of type, the neonatal jaundice treatment market is segmented into phototherapy devices, medications, bilirubin monitoring systems, and others. The phototherapy devices segment dominated the market with the largest revenue share of 46.5% in 2024, driven by their high efficacy, non-invasive nature, and universal adoption in hospitals worldwide. Hospitals and specialty clinics rely heavily on phototherapy as the first-line treatment for neonatal jaundice. The segment benefits from the availability of advanced, energy-efficient, and portable phototherapy units. Increasing clinical awareness, standard treatment guidelines recommending light-based therapy, and the reliability of phototherapy devices for high-risk neonates contribute to its dominance. Phototherapy devices also enjoy high trust among caregivers and healthcare professionals. Regulatory approvals for safety and effectiveness further strengthen adoption. Technological improvements in LED phototherapy systems enhance patient comfort and treatment efficiency. Hospitals continuously upgrade equipment to ensure optimal neonatal care. In addition, the growing prevalence of premature births requiring NICU care sustains demand for phototherapy units. Insurance coverage in developed regions supports consistent usage. Market penetration in emerging economies is increasing as healthcare infrastructure improves. The combination of proven effectiveness, clinical preference, and infrastructure investment positions phototherapy devices as the leading type segment.
The medications segment is expected to witness the fastest CAGR of 19.2% from 2025 to 2032, fueled by rising awareness of drug-based treatments and increasing adoption as adjunct therapy alongside phototherapy. Drug-based therapy offers alternative or complementary options for cases where phototherapy alone is insufficient. Pharmaceutical innovation, including enzyme-based medications, is improving treatment outcomes. Expanding hospital formularies and easier availability of prescription medications boost market growth. Parent and caregiver awareness campaigns further drive adoption. Emerging markets with increasing healthcare expenditure are witnessing rapid uptake. The segment benefits from clinical studies demonstrating efficacy and safety. Combination therapies and customized dosing options appeal to clinicians managing severe or persistent jaundice. The convenience of drug administration supports outpatient and homecare settings. Regulatory approvals and government programs for rare disease management further enhance market penetration. In addition, partnerships between hospitals and pharmaceutical companies improve distribution. Rising neonatal care standards globally support faster adoption. Overall, drug-based therapy represents the fastest-growing type segment in the market.
• By End-User
On the basis of end-user, the neonatal jaundice treatment market is segmented into hospitals, specialty clinics, home healthcare, and others. The hospitals segment held the largest market revenue share of 52.3% in 2024, driven by advanced neonatal care infrastructure, trained clinical staff, and higher patient inflow. Hospitals are the primary care centers for high-risk and premature neonates requiring intensive monitoring and immediate intervention. NICUs and general wards invest in phototherapy devices and monitoring equipment. Hospitals benefit from insurance coverage supporting treatment costs. The presence of specialized neonatal teams ensures standardized treatment protocols. Urban hospitals in developed regions lead in adopting the latest phototherapy technologies. Hospital chains with multiple facilities increase the volume of treatments. Strategic partnerships with device manufacturers ensure equipment availability. Hospitals also drive awareness of drug-based adjunct therapies. Continuous training and guidelines support effective treatment administration. Government initiatives in healthcare infrastructure strengthen hospital capacity. Hospitals act as primary influencers for other end-users adopting neonatal jaundice treatments. Overall, hospitals dominate the end-user segment due to clinical need, volume, and infrastructure.
The home healthcare segment is expected to grow at the fastest CAGR of 17.8% from 2025 to 2032, driven by the rising adoption of home-based phototherapy devices and increasing parental preference for convenient treatment. Telemedicine integration enables remote monitoring by healthcare professionals. Portable phototherapy units allow treatment in non-hospital settings, improving accessibility. Awareness campaigns by healthcare providers and NGOs encourage home-based interventions. Growing comfort with medical devices at home supports adoption. Rising neonatal care awareness among parents fuels demand. Drug-based therapies administered at home further complement treatment. Insurance coverage for homecare devices in some regions promotes market growth. Urbanization and rising disposable incomes increase affordability. Availability of rental or subscription-based phototherapy devices adds convenience. Clinical studies validating homecare efficacy enhance trust. Integration of remote monitoring apps with devices improves adherence. Government and healthcare initiatives supporting homecare increase reach. Overall, home healthcare represents the fastest-growing end-user segment.
• By Treatment Mechanism
On the basis of treatment mechanism, the neonatal jaundice treatment market is segmented into light-based therapy, drug-based therapy, exchange transfusion, and others. The light-based therapy segment dominated with a revenue share of 48.1% in 2024, as phototherapy is the first-line treatment globally. Its high efficacy, minimal side effects, and non-invasive nature make it the preferred method in hospitals and NICUs. Hospitals and clinics invest in high-quality phototherapy units. Guidelines from pediatric and neonatal associations recommend light therapy for most cases. The segment benefits from widespread clinical trust and standardization. LED-based innovations improve energy efficiency and patient comfort. Devices are widely available across developed and emerging regions. Training for healthcare providers ensures correct usage. Hospital procurement and government support facilitate adoption. Light-based therapy reduces the need for invasive procedures. Parental confidence in phototherapy strengthens adherence. Continuous improvements in device technology sustain dominance. High demand in NICUs maintains the leading market position. Overall, light-based therapy is the dominant treatment mechanism segment.
The drug-based therapy segment is expected to witness the fastest CAGR of 18.5% from 2025 to 2032, fueled by growing adoption in adjunct treatment and enzyme-based therapy. Hospitals and specialty clinics increasingly use medications alongside phototherapy. Clinical trials validating efficacy and safety encourage adoption. Rising patient awareness and parental preference for alternative options contribute to growth. Improved access to prescription medications supports expansion. Government initiatives for rare disease management enhance uptake. Pharmaceutical innovation drives development of safer and effective drugs. Homecare adoption of drug therapy complements phototherapy. Urbanization and increasing healthcare access accelerate demand. Insurance coverage facilitates medication availability. Marketing and educational campaigns increase awareness. Combination therapies and hospital partnerships expand reach. Overall, drug-based therapy is the fastest-growing treatment mechanism segment.
• By Application
On the basis of application, the neonatal jaundice treatment market is segmented into neonatal intensive care units (NICUs), general ward, homecare, and others. The NICU segment accounted for the largest market revenue share of 50.2% in 2024, due to higher incidence of jaundice in premature and high-risk infants. NICUs have specialized equipment and trained personnel to administer treatments. High patient inflow ensures consistent demand. Hospitals invest in advanced monitoring systems. Guidelines for NICU care standardize phototherapy and drug-based treatments. Insurance coverage and government funding support NICU treatments. Hospitals adopt latest device technologies in NICUs. Parental trust in NICU care strengthens adoption. Training programs for staff ensure effective treatment administration. NICU capacity expansions increase equipment demand. Strategic hospital partnerships with manufacturers support procurement. Overall, NICUs dominate the application segment.
The homecare segment is expected to witness the fastest CAGR of 16.9% from 2025 to 2032, driven by increasing adoption of portable phototherapy devices and drug-based treatments. Telehealth and remote monitoring improve adherence. Parental preference for at-home care encourages market growth. Rental and subscription models of devices enhance accessibility. Awareness campaigns by healthcare providers promote homecare solutions. Urbanization and rising disposable income enable affordability. Clinical validation of home-based treatments increases trust. Drug therapy for at-home administration complements device usage. Expansion of specialty clinics supports growth. Insurance coverage for homecare treatments in some regions contributes. Technological improvements in compact phototherapy units aid adoption. Government programs supporting neonatal care enhance access. Overall, homecare represents the fastest-growing application segment.
Neonatal Jaundice Treatment Market Regional Analysis
- North America dominated the neonatal jaundice treatment market with the largest revenue share of 41.5% in 2024, driven by advanced healthcare infrastructure, high awareness of neonatal care, and the strong presence of key pharmaceutical and medical device companies.
- The region benefits from well-established hospital networks, high healthcare expenditure, and strong government and private initiatives promoting early diagnosis and treatment of neonatal conditions. Rising incidence of preterm births and associated complications, combined with growing parental awareness of early intervention, is further supporting market growth
- In addition, increasing investment in neonatal care technologies, such as advanced phototherapy devices and bilirubin monitoring systems, and the expansion of specialized neonatal intensive care units (NICUs) are driving the adoption of effective treatment solutions across the region
U.S. Neonatal Jaundice Treatment Market Insight
The U.S. neonatal jaundice treatment market captured the largest share within North America in 2024, driven by substantial growth in hospital-based and outpatient care settings. Increasing adoption of phototherapy devices, bilirubin monitoring systems, and supportive pharmacological treatments, along with innovations from both established companies and specialized neonatal care startups, is fueling market expansion. High physician and parental awareness of neonatal jaundice management further supports sustained growth.
Europe Neonatal Jaundice Treatment Market Insight
The Europe neonatal jaundice treatment market is projected to expand at a considerable CAGR during the forecast period, driven by rising awareness of neonatal health, advanced hospital infrastructure, and strong government support for infant care programs. Countries such as the U.K., Germany, and France are witnessing increasing adoption of modern treatment solutions for newborns, supported by well-established pediatric healthcare networks. Expansion of neonatal intensive care units (NICUs) and improved access to prescription therapies are significant growth drivers.
U.K. Neonatal Jaundice Treatment Market Insight
The U.K. neonatal jaundice treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by rising awareness of neonatal jaundice complications and increasing adoption of effective treatments in hospitals and specialty clinics. Government initiatives promoting early screening and treatment, alongside robust healthcare infrastructure, support the growing demand for phototherapy and pharmacological solutions.
Germany Neonatal Jaundice Treatment Market Insight
The Germany neonatal jaundice treatment market is expected to expand at a significant CAGR, supported by strong neonatal care services, advanced hospital systems, and rising parental awareness of early intervention benefits. The increasing prevalence of preterm births and associated neonatal complications is further boosting demand for effective treatment solutions.
Asia-Pacific Neonatal Jaundice Treatment Market Insight
The Asia-Pacific neonatal jaundice treatment market is expected to grow at the fastest CAGR of 23.5% during 2025–2032, driven by increasing urbanization, rising healthcare expenditure, expanding neonatal care facilities, and growing awareness of the benefits of early treatment in countries such as China, India, and Japan. The rising number of hospital deliveries, government initiatives for infant health, and improved access to modern phototherapy and pharmacological treatments are fueling rapid adoption across the region.
Japan Neonatal Jaundice Treatment Market Insight
The Japan neonatal jaundice treatment market is gaining momentum due to rising neonatal care awareness, a strong focus on infant health, and the adoption of advanced phototherapy and pharmacological treatments. High standards of hospital care and increasing parental knowledge about early intervention contribute to steady growth in the country.
China Neonatal Jaundice Treatment Market Insight
The China neonatal jaundice treatment market accounted for the largest market revenue share in Asia-Pacific in 2024, driven by expanding neonatal healthcare infrastructure, a growing middle class, rising awareness of early treatment benefits, and increasing hospital and outpatient care facilities. The country’s focus on improving infant health outcomes, combined with accessibility to both phototherapy devices and pharmacological solutions, is significantly propelling market expansion.
Neonatal Jaundice Treatment Market Share
The Neonatal Jaundice Treatment industry is primarily led by well-established companies, including:
- GE Healthcare (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- Natus Medical Incorporated (U.S.)
- Drägerwerk AG & Co. KGaA (Germany)
- Atom Medical Corporation (Japan)
- Bilimetrix (U.S.)
- BiliSoft (U.S.)
- Phoenix Medical Systems (India)
- Biofotonik (Sweden)
- QED Scientific (U.K.)
- Firefly Design + Communications, Inc. (U.K.)
- Nareena Lifesciences Private Limited (India)
Latest Developments in Neonatal Jaundice Treatment Market
- In March 2021, researchers at Yokohama National University and Yokohama City University developed the first wearable device capable of continuously monitoring bilirubin levels and vital signs in newborns. This innovation aims to provide real-time data to healthcare providers, potentially reducing the need for invasive procedures and improving early detection of neonatal jaundice
- In November 2022, a portable, battery-operated phototherapy device designed for treating neonatal jaundice won the TropMed2022 Innovations Pitch Competition. This device offers a non-invasive, cost-effective solution for jaundice treatment, particularly beneficial in low-resource settings where access to traditional hospital-based phototherapy may be limited
- In July 2024, Konica Minolta and Picterus AS announced a collaboration to enhance technologies aimed at protecting newborns from severe consequences of jaundice. This partnership focuses on improving diagnostic and treatment methods, potentially leading to more effective and accessible care for neonates affected by jaundice
- In August 2024, a study published in Nature highlighted the feasibility of administering phototherapy for neonatal jaundice at home. The research suggests that with proper training and equipment, home-based phototherapy can be a safe and effective alternative to hospital treatment, offering convenience and reducing healthcare costs
- In July 2025, Anshumaan Karna, a student from IIIT Naya Raipur, developed an AI-powered smartphone application capable of non-invasively detecting neonatal jaundice by predicting bilirubin levels. The prototype is currently under review at IIT Kharagpur, indicating potential for further development and implementation in clinical settings
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.