Global Neuromyelitis Optica Spectrum Disorder Nmosd Market
Market Size in USD Million
CAGR :
% 
 USD
405.90 Million
USD
651.84 Million
2021
2029
USD
405.90 Million
USD
651.84 Million
2021
2029
| 2022 –2029 | |
| USD 405.90 Million | |
| USD 651.84 Million | |
|
|
|
|
Market Analysis and Size
Neuromyelitis optica spectrum disorder (NMOSD) is a chronic brain and spinal cord ailment characterized by inflammation of the optic nerve (optic neuritis) and spinal cord inflammation (myelitis). It was once thought to be a monophasic condition, with periods of inflammation of one or both optic nerves and the spinal cord lasting only a few days or weeks and no recurrence beyond the initial episode. According to new research, most individuals who meet the existing criteria for NMOSD have many attacks separated by periods of remission. This condition is also termed as Devic disease.
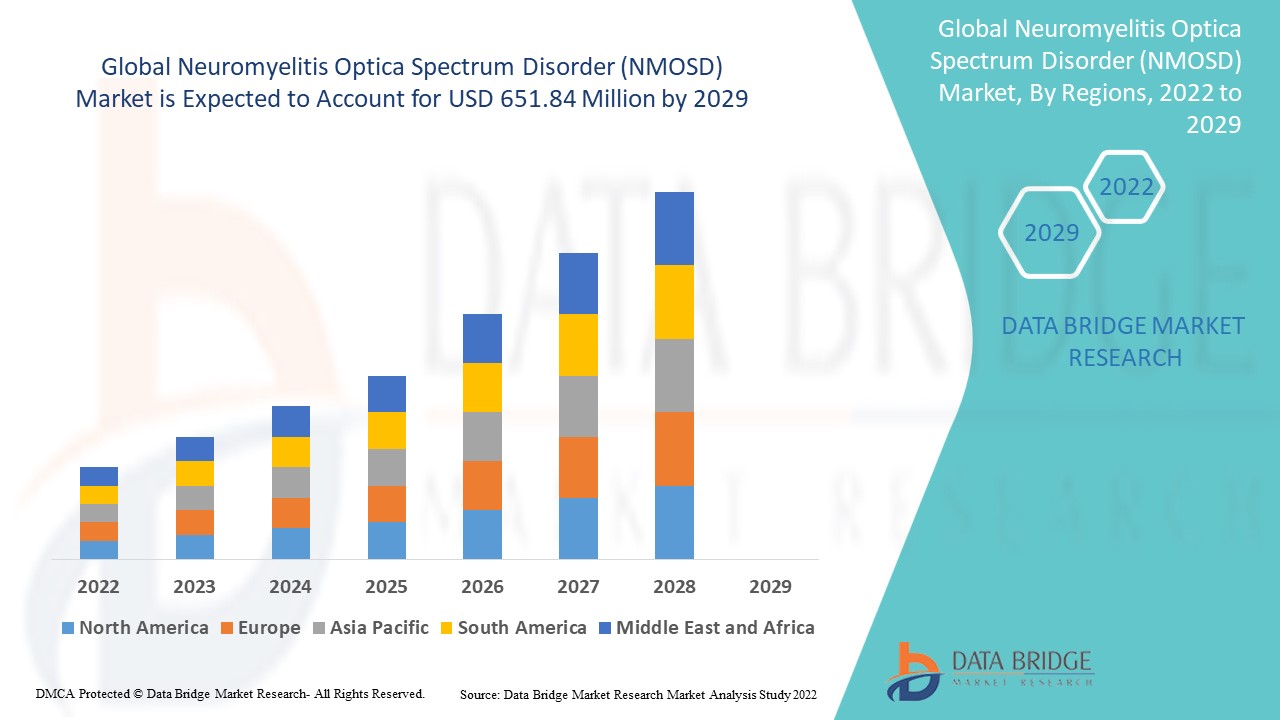
Data Bridge Market Research analyses that the neuromyelitis optica spectrum disorder (NMOSD) market was valued at USD 405.9 million in 2021 and is expected to reach USD 651.84 million by 2029, registering a CAGR of 6.10% during the forecast period of 2022 to 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Neuromyelitis Optica Spectrum Disorder with Aquaporin-4 Antibodies, Neuromyelitis Optica Spectrum Disorder without Aquaporin-4 Antibodies), Treatment Type (Medication, Plasma Exchange Therapy, Immunoglobulin Therapy), Route of Administration (Oral, Parenteral, Others), Dosage Form (Tablets, Injections, Others), Diagnosis (Imaging Tests, Blood Tests, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd.(Israel), Sanofi (France), Pfizer Inc. (U.S.), GlaxoSmithKline plc (U.K.), Novartis AG (Switzerland), Zydus Cadila (India), Boehringer Ingelheim International GmbH. (Germany), Apotex Inc. (Canada), AstraZeneca (U.K.), Horizon Therapeutics PLC (Ireland), Johnson & Johnson Private Limited (U.S.), Bayer AG (Germany), Sun Pharmaceutical Industries Ltd. (India), LEO Pharma A/S (Denmark), Bausch Health Companies Inc. (Canada), Allergan (Ireland), Eli Lilly and Company (U.S.), Aurobindo Pharma (India), Lupin (India) |
|
Market Opportunities |
|
Market Definition
NMOSD (neuromyelitis optica spectrum disorder) is a central nervous system inflammatory ailment that was previously known as Devic disease or neuromyelitis optica (NMO). It's a devastating, life-long disease marked by spinal cord inflammation and optic nerve. Paralysis, muscle weakness, and blindness are common neuromyelitis optica spectrum disease symptoms. Non-Caucasian women are more likely to have neuromyelitis optica spectrum disorder. Genentech's Satralizumab is currently through a phase III clinical trial. It's a humanized monoclonal antibody that targets the IL-6 receptor in clinical trials. It's being tested to examine whether it can help with neuromyelitis optica spectrum disorder (NMOSD).
Neuromyelitis Optica Spectrum Disorder (NMOSD) Market Dynamics
Drivers
- Increasing investment for healthcare infrastructure
Another significant factor influencing the growth rate of neuromyelitis optica spectrum disorder (NMOSD) market is the rising healthcare expenditure which helps in improving its infrastructure. Along with this, for better patient outcomes, manufacturers in the market for treating optic nerve disorders are teaching healthcare professionals about drug-specific factors of topical ocular corticosteroids, such as AE profile, potency, and patient-specific administration needs. Also, various government organizations aims to improve the healthcare infrastructure by increasing funding and this will further influence the market dynamics.
- High prevalence of multiple sclerosis (MS)
The high prevalence of multiple sclerosis is expected to act as a major driver for neuromyelitis optica spectrum disorder (NMOSD) market's growth. MS is an autoimmune disease in which the immune system assaults healthy bodily components, resulting in eyesight loss or decrease in more than half of the cases. As a result, manufacturers are expanding their production capacity in order to develop immunosuppressive medications that improve patients' quality of life. In September 2019, Roche, a Swiss multinational healthcare company, announced the results of clinical studies for Ocrevus, an immunosuppressive medicine, to better understand its impact in relapsing and progressive MS.
Furthermore, rising initiatives by public and private organizations to spread awareness and surging geriatric population will expand the neuromyelitis optica spectrum disorder (NMOSD) market. Additionally, high incidence of neuromyelitis optica spectrum disorder (NMOSD) and the sedentary lifestyle of people will result in the expansion of neuromyelitis optica spectrum disorder (NMOSD) market.
Opportunities
- Increase in the number of research and development activities
Moreover, the market's growth is fueled by an increase in the number of research and development activities of medication for symptomatic treatment of osteoarthritis. This will provide beneficial opportunities for market growth for neuromyelitis optica spectrum disorder (NMOSD). The pivotal phase III study of the experimental medication satralizumab for the treatment of neuromyelitis optica spectrum condition was announced by F. Hoffmann-La Roche Ltd in December 2019. The phase III clinical trial for neuromyelitis optica spectrum condition with inebilizumab (Anti-CD19 mAb) was finished in 2019. Viela Bio is the provider.
- Launch of new products and therapies
The increasing number of new product launches and therapies for the treatment of the neuromyelitis optica spectrum disorder (NMOSD) is anticipated to create new market opportunities during the forecast period of 2022 to 2029. The advent of new therapies for the treatment of neuromyelitis optica spectrum syndrome poses a potential market opportunity. Bevacizumab and Alpha1-antitrypsin and Rituximab and Eculizumab are two new treatments in progression for the acute phase and prophylaxis, respectively.
Moreover, rising investment for the development of advanced technologies and increase in the number of emerging markets will further provide beneficial opportunities for the neuromyelitis optica spectrum disorder (NMOSD) market growth during the forecast period.
Restraints/Challenges
On the other hand, high cost associated with the treatment will obstruct the growth rate of market. The dearth of skilled professionals and lack of healthcare infrastructure in developing economies will challenge the neuromyelitis optica spectrum disorder (NMOSD) market. Additionally, strict regulatory policies and lack of awareness among people will restrain and further impede the growth rate of the market during the forecast period of 2022-2029.
This neuromyelitis optica spectrum disorder (NMOSD) market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the neuromyelitis optica spectrum disorder (NMOSD) market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Patient Epidemiology Analysis
The prevalence of NMOSD is comparable over the world, rarely exceeding 5 per 100,000. 4 The proportion of MS and NMOSD patients is more a function of MS prevalence variability than NMOSD prevalence variability. Females suffer more than males (3:1 in MS vs. 9:1 in NMOSD). The median age at presentation is 39 years, however children and the elderly account for 18% of cases. It is generally thought to be a non-familial disorder.
Neuromyelitis optica spectrum disorder (NMOSD) market also provides you with detailed market analysis for patient analysis, prognosis and cures. Prevalence, incidence, mortality, adherence rates are some of the data variables that are available in the report. Direct or indirect impact analyses of epidemiology to market growth are analysed to create a more robust and cohort multivariate statistical model for forecasting the market in the growth period.
Recent Development
- In August 2020, the U.S. Food and Drug Administration (FDA) had approved Enspryng (satralizumab-mwge) for treating neuromyelitis optica spectrum disorder (NMOSD) in adults. Enspryng was given fast track designation, which expedites the development and review of drugs that are intended to treat a serious condition and show promise of meeting an unmet medical need. The drug was also designated as an orphan drug, which provides financial incentives to help and stimulate drug development for rare disorders.
Global Neuromyelitis Optica Spectrum Disorder (NMOSD) Market Scope
The neuromyelitis optica spectrum disorder (NMOSD) market is segmented on the basis of type, treatment type, diagnosis, dosage form, route of administration, end-users and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Neuromyelitis Optica Spectrum Disorder with Aquaporin-4 Antibodies
- Neuromyelitis Optica Spectrum Disorder without Aquaporin-4 Antibodies
Treatment Type
- Medication
- Immunosuppressive Agent
- C5 Protein Inhibitor
- Monoclonal Antibodies
- Corticosteroids
- Others
- Plasma Exchange Therapy
- Immunoglobulin Therapy
- Diagnosis
- Imaging Tests
- Magnetic resonance imaging (MRI)
- Others
- Blood Tests
- Others
Dosage form
- Tablets
- Injections
- Others
Route of Administration
- Oral
- Parenteral
- Others
End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Neuromyelitis Optica Spectrum Disorder (NMOSD) Market Regional Analysis/Insights
The neuromyelitis optica spectrum disorder (NMOSD) market is analysed and market size insights and trends are provided by country, type, treatment type, diagnosis, dosage form, route of administration, end-users and distribution channel as referenced above.
The countries covered in the neuromyelitis optica spectrum disorder (NMOSD) market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
North America dominates the neuromyelitis optica spectrum disorder (NMOSD) market because of the launch of new treatment options in this region. Additionally, rising healthcare expenditure along with the availability of treatment options will further propel the market's growth rate in this region.
Asia-Pacific is expected to grow during the forecast period due to presence of generic manufacturers in this region. Also, development of healthcare infrastructure and rising geriatric population will further propel the market's growth rate in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Neuromyelitis Optica Spectrum Disorder (NMOSD) Market Share Analysis
The Neuromyelitis optica spectrum disorder (NMOSD) market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to neuromyelitis optica spectrum disorder (NMOSD) market.
Some of the major players operating in the neuromyelitis optica spectrum disorder (NMOSD) market are:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Mylan N.V. (U.S.)
- Teva Pharmaceutical Industries Ltd.(Israel)
- Sanofi (France), Pfizer Inc. (U.S.)
- GlaxoSmithKline plc (U.K.)
- Novartis AG (Switzerland)
- Zydus Cadila (India)
- Boehringer Ingelheim International GmbH. (Germany)
- Apotex Inc. (Canada)
- AstraZeneca (U.K.)
- Horizon Therapeutics PLC (Ireland)
- Johnson & Johnson Private Limited (U.S.)
- Bayer AG (Germany)
- Sun Pharmaceutical Industries Ltd. (India)
- LEO Pharma A/S (Denmark)
- Bausch Health Companies Inc. (Canada)
- Allergan (Ireland)
- Eli Lilly and Company (U.S.)
- Aurobindo Pharma (India)
- Lupin (India)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 EPIDEMIOLOGY BASED MODEL
2.2.11 VENDOR SHARE ANALYSIS
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 EPIDEMIOLOGY
7 INDUSTRY INSIGHTS
8 REGULATORY SCENARIO
9 PIPELINE ANALYSIS
9.1 PHASE III CANDIDATES
9.2 PHASE II CANDIDATES
9.3 PHASE I CANDIDATES
9.4 OTHERS (PRE-CLINICAL AND RESEARCH)
10 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY DISEASE TYPE
10.1 OVERVIEW
10.2 RELAPSING FORM
10.3 MONOPHASIC FORM
11 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY ANTIBODIES
11.1 OVERVIEW
11.2 ANTI-AQP4+ VARIANTS
11.3 ANTI-MOG+ VARIANTS
12 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY TREATEMT
12.1 OVERVIEW
12.2 DRUG
12.2.1 MONOCLONAL ANTIBODY
12.2.1.1. ECULIZUMAB
12.2.1.2. INEBILIZUMAB
12.2.1.3. SATRALIZUMAB
12.2.1.4. OTHER
12.2.2 IMMUNOSUPPRESSIVE
12.2.2.1. AZATHIOPRINE
12.2.2.2. MYCOPHENOLATE
12.2.2.3. RITUXIMAB
12.2.2.4. METHOTREXATE
12.2.2.5. MITOXANTRONE
12.2.2.6. ECULIZUMAB
12.2.2.7. TOCILIZUMAB
12.2.2.8. OTHER
12.2.2.9. CORTICOSTEROIDS
12.2.2.9.1. METHYLPREDNISOLONE
12.2.2.9.2. PREDNISONE
12.2.2.9.3. OTHERS
12.2.3 ANTI-EPILEPTIC MEDICATIONS
12.2.3.1. GABAPENTIN
12.2.3.2. CARBAMAZEPINE
12.2.3.3. OTHERS
12.2.4 ANTI-SPASMODICS
12.2.4.1. BACLOFEN
12.2.4.2. TIZANIDINE
12.2.4.3. OTHERS
12.2.5 ANTI-DEPRESSANTS
12.2.5.1. AMITRIPTYLINE
12.2.5.2. DULOXETINE
12.2.5.3. OTHERS
12.2.6 ANALGESICS
12.2.6.1. TRAMADOL
12.2.6.2. OPIATES
12.2.6.3. OTHERS
12.2.7 OTHERS
12.3 PLASMA THERAPY
12.4 DIAGNOSIS
12.4.1 MRI
12.4.2 ANTIBODY TEST
12.4.3 LUMBAR PUNCTURE (SPINAL TAP)
12.4.4 STIMULI RESPONSE TEST
12.4.5 OTHERS
12.5 OTHER
13 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY DRUG TYPE
13.1 OVERVIEW
13.2 BRANDED
13.2.1 SOLIRIS
13.2.2 UPLIZNA
13.2.3 ENSPRYNG
13.2.4 OTHER
13.3 GENERICS
14 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY SYMPTOMS
14.1 OVERVIEW
14.2 OPTIC NEURITIS
14.3 ACUTE MYELITIS
14.4 AREA POSTREMA SYNDROME
14.5 ACUTE BRAINSTEM SYNDROME
14.6 SYMPTOMATIC NARCOLEPSY/ACUTE DIENCEPHALIC CLINICAL SYNDROME
14.7 SYMPTOMATIC CEREBRAL SYNDROME WITH NSMOD-TYPICAL BRAIN LESION
14.8 OTHERS
15 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY POPULATION TYPE
15.1 OVERVIEW
15.2 PEDIATRIC
15.3 ADULTS
16 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY ROUTE OF ADMINISTRATION
16.1 OVERVIEW
16.2 ORAL
16.2.1 TABLETS
16.2.2 SUSPENSION
16.2.3 OTHERS
16.3 PARENTERAL
16.3.1 INTRAVENOUS
16.3.2 SUBCUTANEOUS
16.3.3 OTHERS
16.4 OTHERS
17 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITALS
17.3 SPECIALTY CLINICS
17.4 HOME HEALTHCARE
17.5 OTHERS
18 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY DISTRIBUTION CHANNEL
18.1 OVERVIEW
18.2 HOSPITAL PHARMACIES
18.3 RETAIL PHARMACIES
18.4 ONLINE PHARMACIES
18.5 OTHERS
19 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, COMPANY LANDSCAPE
19.1 COMPANY SHARE ANALYSIS: GLOBAL
19.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
19.3 COMPANY SHARE ANALYSIS: EUROPE
19.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
19.5 MERGERS & ACQUISITIONS
19.6 NEW PRODUCT DEVELOPMENT & APPROVALS
19.7 EXPANSIONS
19.8 REGULATORY CHANGES
19.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
20 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, BY GEOGRAPHY
GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
20.1 NORTH AMERICA
20.1.1 U.S.
20.1.2 CANADA
20.1.3 MEXICO
20.2 EUROPE
20.2.1 GERMANY
20.2.2 U.K.
20.2.3 ITALY
20.2.4 FRANCE
20.2.5 SPAIN
20.2.6 RUSSIA
20.2.7 SWITZERLAND
20.2.8 TURKEY
20.2.9 BELGIUM
20.2.10 NETHERLANDS
20.2.11 DENMARK
20.2.12 SWEDEN
20.2.13 POLAND
20.2.14 NORWAY
20.2.15 FINLAND
20.2.16 REST OF EUROPE
20.3 ASIA-PACIFIC
20.3.1 JAPAN
20.3.2 CHINA
20.3.3 SOUTH KOREA
20.3.4 INDIA
20.3.5 SINGAPORE
20.3.6 THAILAND
20.3.7 INDONESIA
20.3.8 MALAYSIA
20.3.9 PHILIPPINES
20.3.10 AUSTRALIA
20.3.11 NEW ZEALAND
20.3.12 VIETNAM
20.3.13 TAIWAN
20.3.14 REST OF ASIA-PACIFIC
20.4 SOUTH AMERICA
20.4.1 BRAZIL
20.4.2 ARGENTINA
20.4.3 REST OF SOUTH AMERICA
20.5 MIDDLE EAST AND AFRICA
20.5.1 SOUTH AFRICA
20.5.2 EGYPT
20.5.3 BAHRAIN
20.5.4 UNITED ARAB EMIRATES
20.5.5 KUWAIT
20.5.6 OMAN
20.5.7 QATAR
20.5.8 SAUDI ARABIA
20.5.9 REST OF MEA
20.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
21 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, SWOT AND DBMR ANALYSIS
22 GLOBAL NEUROMYELITIS OPTICA SPECTRUM DISORDER (NMOSD) MARKET, COMPANY PROFILE
22.1 ALEXION PHARMACEUTICALS, INC.
22.1.1 COMPANY OVERVIEW
22.1.2 REVENUE ANALYSIS
22.1.3 GEOGRAPHIC PRESENCE
22.1.4 PRODUCT PORTFOLIO
22.1.5 RECENT DEVELOPMENTS
22.2 VIELA BIO.( HORIZON THERAPEUTICS PLC)
22.2.1 COMPANY OVERVIEW
22.2.2 REVENUE ANALYSIS
22.2.3 GEOGRAPHIC PRESENCE
22.2.4 PRODUCT PORTFOLIO
22.2.5 RECENT DEVELOPMENTS
22.3 F. HOFFMANN-LA ROCHE LTD
22.3.1 COMPANY OVERVIEW
22.3.2 REVENUE ANALYSIS
22.3.3 GEOGRAPHIC PRESENCE
22.3.4 PRODUCT PORTFOLIO
22.3.5 RECENT DEVELOPMENTS
22.4 AMNEAL PHARMACEUTICALS LLC
22.4.1 COMPANY OVERVIEW
22.4.2 REVENUE ANALYSIS
22.4.3 GEOGRAPHIC PRESENCE
22.4.4 PRODUCT PORTFOLIO
22.4.5 RECENT DEVELOPMENTS
22.5 ZYDUS PHARMACEUTICALS, INC.
22.5.1 COMPANY OVERVIEW
22.5.2 REVENUE ANALYSIS
22.5.3 GEOGRAPHIC PRESENCE
22.5.4 PRODUCT PORTFOLIO
22.5.5 RECENT DEVELOPMENTS
22.6 FRESENIUS KABI USA
22.6.1 COMPANY OVERVIEW
22.6.2 REVENUE ANALYSIS
22.6.3 GEOGRAPHIC PRESENCE
22.6.4 PRODUCT PORTFOLIO
22.6.5 RECENT DEVELOPMENTS
22.7 CHORD THERAPEUTICS SA
22.7.1 COMPANY OVERVIEW
22.7.2 REVENUE ANALYSIS
22.7.3 GEOGRAPHIC PRESENCE
22.7.4 PRODUCT PORTFOLIO
22.7.5 RECENT DEVELOPMENTS
22.8 ACCORD-HEALTHCARE
22.8.1 COMPANY OVERVIEW
22.8.2 REVENUE ANALYSIS
22.8.3 GEOGRAPHIC PRESENCE
22.8.4 PRODUCT PORTFOLIO
22.8.5 RECENT DEVELOPMENTS
22.9 GENENTECH INC
22.9.1 COMPANY OVERVIEW
22.9.2 REVENUE ANALYSIS
22.9.3 GEOGRAPHIC PRESENCE
22.9.4 PRODUCT PORTFOLIO
22.9.5 RECENT DEVELOPMENTS
22.1 HORIZON THERAPEUTICS PLC
22.10.1 COMPANY OVERVIEW
22.10.2 REVENUE ANALYSIS
22.10.3 GEOGRAPHIC PRESENCE
22.10.4 PRODUCT PORTFOLIO
22.10.5 RECENT DEVELOPMENTS
22.11 MYLAN N.V.
22.11.1 COMPANY OVERVIEW
22.11.2 REVENUE ANALYSIS
22.11.3 GEOGRAPHIC PRESENCE
22.11.4 PRODUCT PORTFOLIO
22.11.5 RECENT DEVELOPMENTS
22.12 ASPEN PHARAMACRE PYT LTD
22.12.1 COMPANY OVERVIEW
22.12.2 REVENUE ANALYSIS
22.12.3 GEOGRAPHIC PRESENCE
22.12.4 PRODUCT PORTFOLIO
22.12.5 RECENT DEVELOPMENTS
22.13 SANIS HEALTHCARE INC
22.13.1 COMPANY OVERVIEW
22.13.2 REVENUE ANALYSIS
22.13.3 GEOGRAPHIC PRESENCE
22.13.4 PRODUCT PORTFOLIO
22.13.5 RECENT DEVELOPMENTS
22.14 AMGEN INC
22.14.1 COMPANY OVERVIEW
22.14.2 REVENUE ANALYSIS
22.14.3 GEOGRAPHIC PRESENCE
22.14.4 PRODUCT PORTFOLIO
22.14.5 RECENT DEVELOPMENTS
22.15 TEVA PHARMACEUTICAL INDUSTRIES LTD.
22.15.1 COMPANY OVERVIEW
22.15.2 REVENUE ANALYSIS
22.15.3 GEOGRAPHIC PRESENCE
22.15.4 PRODUCT PORTFOLIO
22.15.5 RECENT DEVELOPMENTS
22.16 PFIZER INC.
22.16.1 COMPANY OVERVIEW
22.16.2 REVENUE ANALYSIS
22.16.3 GEOGRAPHIC PRESENCE
22.16.4 PRODUCT PORTFOLIO
22.16.5 RECENT DEVELOPMENTS
22.17 GLAXOSMITHKLINE PLC
22.17.1 COMPANY OVERVIEW
22.17.2 REVENUE ANALYSIS
22.17.3 GEOGRAPHIC PRESENCE
22.17.4 PRODUCT PORTFOLIO
22.17.5 RECENT DEVELOPMENTS
22.18 NOVARTIS AG
22.18.1 COMPANY OVERVIEW
22.18.2 REVENUE ANALYSIS
22.18.3 GEOGRAPHIC PRESENCE
22.18.4 PRODUCT PORTFOLIO
22.18.5 RECENT DEVELOPMENTS
22.19 APOTEX INC.
22.19.1 COMPANY OVERVIEW
22.19.2 REVENUE ANALYSIS
22.19.3 GEOGRAPHIC PRESENCE
22.19.4 PRODUCT PORTFOLIO
22.19.5 RECENT DEVELOPMENTS
22.2 SUN PHARMACEUTICAL INDUSTRIES LTD.
22.20.1 COMPANY OVERVIEW
22.20.2 REVENUE ANALYSIS
22.20.3 GEOGRAPHIC PRESENCE
22.20.4 PRODUCT PORTFOLIO
22.20.5 RECENT DEVELOPMENTS
22.21 ANATARES PHARMA
22.21.1 COMPANY OVERVIEW
22.21.2 REVENUE ANALYSIS
22.21.3 GEOGRAPHIC PRESENCE
22.21.4 PRODUCT PORTFOLIO
22.21.5 RECENT DEVELOPMENTS
22.22 LUPIN
22.22.1 THE COMPANY OVERVIEW
22.22.2 REVENUE ANALYSIS
22.22.3 GEOGRAPHIC PRESENCE
22.22.4 PRODUCT PORTFOLIO
22.22.5 RECENT DEVELOPMENTS
22.23 HIKMA PHARAMACEUTICALS PLC
22.23.1 THE COMPANY OVERVIEW
22.23.2 REVENUE ANALYSIS
22.23.3 GEOGRAPHIC PRESENCE
22.23.4 PRODUCT PORTFOLIO
22.23.5 RECENT DEVELOPMENTS
22.24 STRIDES PHARMA INC
22.24.1 THE COMPANY OVERVIEW
22.24.2 REVENUE ANALYSIS
22.24.3 GEOGRAPHIC PRESENCE
22.24.4 PRODUCT PORTFOLIO
22.24.5 RECENT DEVELOPMENTS
22.25 SEBELA PHARAMACEUTICALS
22.25.1 THE COMPANY OVERVIEW
22.25.2 REVENUE ANALYSIS
22.25.3 GEOGRAPHIC PRESENCE
22.25.4 PRODUCT PORTFOLIO
22.25.5 RECENT DEVELOPMENTS
22.26 CONCORD BIOTECH
22.26.1 THE COMPANY OVERVIEW
22.26.2 REVENUE ANALYSIS
22.26.3 GEOGRAPHIC PRESENCE
22.26.4 PRODUCT PORTFOLIO
22.26.5 RECENT DEVELOPMENTS
22.27 JUBILANT CADISTA PHARMACEUTICALS INC
22.27.1 THE COMPANY OVERVIEW
22.27.2 REVENUE ANALYSIS
22.27.3 GEOGRAPHIC PRESENCE
22.27.4 PRODUCT PORTFOLIO
22.27.5 RECENT DEVELOPMENTS
*NOTE: COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
23 RELATED REPORTS
24 CONCLUSION
25 QUESTIONNAIRE
26 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












