Global Ocular Fluorometholone Market
Market Size in USD Million
CAGR :
% 
 USD
12.71 Million
USD
19.95 Million
2024
2032
USD
12.71 Million
USD
19.95 Million
2024
2032
| 2025 –2032 | |
| USD 12.71 Million | |
| USD 19.95 Million | |
|
|
|
|
Ocular Fluorometholone Market Size
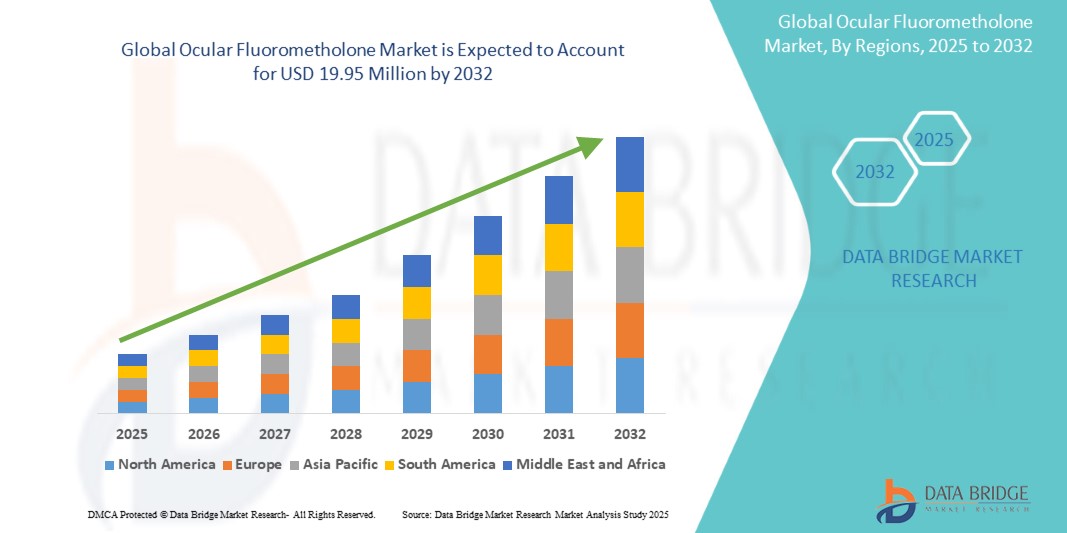
- The global ocular fluorometholone market size was valued at USD 12.71 million in 2024 and is expected to reach USD 19.95 million by 2032, at a CAGR of 5.80% during the forecast period
- The market growth is largely fueled by the increasing prevalence of ocular inflammatory conditions, rising awareness of eye care, and the growing adoption of advanced ophthalmic therapies across hospitals, clinics, and eye care centers
- Furthermore, the rising demand for effective, safe, and easy-to-administer corticosteroid solutions for the treatment of ocular inflammation and post-operative eye conditions is accelerating the uptake of ocular fluorometholone products, thereby significantly boosting the industry's growth globally
Ocular Fluorometholone Market Analysis
- Ocular Fluorometholone, a corticosteroid used for the treatment of ocular inflammation, post-operative eye conditions, and other inflammatory eye disorders, is increasingly vital in modern ophthalmic care due to its effectiveness, safety profile, and ease of administration across hospitals, clinics, and specialized eye care centers
- The escalating demand for ocular fluorometholone is primarily fueled by the rising prevalence of ocular inflammatory conditions, growing awareness of eye health, and the increasing preference for clinically proven corticosteroid therapies for both acute and chronic eye inflammation
- North America dominated the ocular fluorometholone market with the largest revenue share of 41.2% in 2024, driven by advanced healthcare infrastructure, early adoption of innovative ophthalmic therapies, high patient awareness, and strong presence of key pharmaceutical companies. The U.S. is witnessing substantial growth in ocular fluorometholone usage across hospitals and clinics, supported by ongoing clinical research and post-operative treatment protocols
- Asia-Pacific is expected to be the fastest-growing region in the ocular fluorometholone market during the forecast period, with a CAGR of 23.5%, due to increasing urbanization, rising disposable incomes, expanding healthcare infrastructure, and growing access to specialized eye care in countries such as China, India, and Japan
- The 0.1% Fluorometholone Eye Drops segment dominated the ocular fluorometholone market with a revenue share of 61.3% in 2024, owing to its higher potency and effectiveness in managing moderate to severe ocular inflammation
Report Scope and Ocular Fluorometholone Market Segmentation
|
Attributes |
Ocular Fluorometholone Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Ocular Fluorometholone Market Trends
Growing Adoption Driven by Advanced Formulations and Targeted Therapy
- A significant and accelerating trend in the global ocular fluorometholone market is the development and adoption of advanced formulations, including ophthalmic suspensions, drops, and ointments, which enhance drug delivery and patient compliance for ocular inflammatory conditions
- For instance, companies are increasingly introducing preservative-free formulations and combination therapies that improve efficacy while minimizing side effects, enabling safer long-term management of conditions such as uveitis and post-operative ocular inflammation
- Advanced drug delivery technologies in ocular fluorometholone enable targeted action, reducing systemic exposure and enhancing treatment outcomes. Novel suspension and controlled-release formulations ensure precise dosing, improving therapeutic effectiveness and patient adherence
- The expansion of these innovative formulations facilitates broader access to ophthalmic care, as patients can receive effective anti-inflammatory therapy in outpatient and home settings. This trend is encouraging pharmaceutical companies to invest in research and development for new and improved ocular fluorometholone products
- The rising demand for formulations that offer convenience, minimal side effects, and targeted therapeutic action is reshaping treatment protocols for ocular inflammation. Consequently, leading companies are focusing on introducing optimized suspensions, drop concentrations, and post-surgical regimens to meet evolving patient and physician needs
- The market growth for ocular fluorometholone is being fueled by both rising prevalence of ocular inflammatory disorders and the increasing preference among ophthalmologists for proven, safe, and patient-friendly corticosteroid therapies across hospitals, clinics, and specialized eye care centers
Ocular Fluorometholone Market Dynamics
Driver
Growing Need Due to Rising Prevalence of Ocular Inflammatory Disorders
- The increasing prevalence of ocular inflammatory conditions among patients, coupled with the growing awareness of effective ophthalmic treatments, is a significant driver for the heightened demand for Ocular Fluorometholone
- For instance, pharmaceutical companies are actively introducing improved formulations of Ocular Fluorometholone, such as preservative-free drops and targeted suspensions, to enhance efficacy and patient safety. Such strategies by key companies are expected to drive the market growth in the forecast period
- As healthcare providers and patients become more aware of the risks associated with untreated ocular inflammation, the demand for corticosteroid solutions that effectively manage conditions such as uveitis, post-operative inflammation, and allergic conjunctivitis is increasing
- Furthermore, the rising adoption of ocular fluorometholone in hospitals, ophthalmic clinics, and specialized eye care centers is improving patient outcomes by offering precise, safe, and convenient therapeutic options
- The convenience of easy-to-administer drops, suitability for both acute and chronic conditions, and compatibility with outpatient treatment protocols are key factors propelling the adoption of ocular fluorometholone globally. The expansion of patient-friendly formulations further contributes to market growth
Restraint/Challenge
Concerns Regarding Side Effects and High Treatment Costs
- Concerns regarding potential side effects of corticosteroid treatments, including increased intraocular pressure and risk of glaucoma, pose a significant challenge to broader market adoption. Patients and physicians may be cautious about long-term use without proper monitoring
- For instance, high-profile clinical studies highlighting corticosteroid-induced ocular complications have made some practitioners more selective in prescribing ocular fluorometholone
- Addressing these concerns through improved formulations, such as low-dose or preservative-free variants, and patient education on safe usage is crucial for building trust and encouraging adoption. In addition, the relatively high cost of advanced ocular fluorometholone formulations compared to generic corticosteroids can be a barrier, particularly in developing regions or among price-sensitive patients
- While generic alternatives are gradually increasing market accessibility, premium formulations with enhanced safety, efficacy, and convenience often come with higher price points
- Overcoming these challenges through ongoing clinical research, development of cost-effective formulations, and educational initiatives for both physicians and patients will be vital for sustained growth of the ocular fluorometholone market
Ocular Fluorometholone Market Scope
The market is segmented on the basis of product type, application, and distribution channel.
• By Product Type
On the basis of product type, the ocular fluorometholone market is segmented into 0.1% Fluorometholone Eye Drops and 0.02% Fluorometholone Eye Drops. The 0.1% Fluorometholone Eye Drops segment dominated the market with a revenue share of 61.3% in 2024, owing to its higher potency and effectiveness in managing moderate to severe ocular inflammation. Ophthalmologists prefer this concentration for treating postoperative inflammation, uveitis, and other ocular conditions requiring strong corticosteroid therapy. The dominance is reinforced by its well-established safety profile, clinical acceptance, and ability to deliver rapid symptom relief. Patients benefit from targeted anti-inflammatory effects with minimal systemic exposure. The segment also benefits from hospital recommendations and inclusion in post-surgical treatment protocols. Its widespread availability across hospitals and retail pharmacies further consolidates its market position.
The 0.02% Fluorometholone Eye Drops segment is expected to witness the fastest CAGR of 18.5% from 2025 to 2032, driven by the growing demand for milder corticosteroid formulations suitable for sensitive eyes, pediatric patients, and long-term therapy. Physicians increasingly prefer lower-concentration drops to minimize potential side effects such as increased intraocular pressure. Rising awareness among patients about safer steroid use is boosting adoption. This segment is also gaining traction in outpatient clinics and self-care settings due to convenience and ease of use. Pharmaceutical companies are launching preservative-free versions to further enhance safety. The trend toward personalized treatment is supporting the growth of this subsegment.
• By Application
On the basis of application, the ocular fluorometholone market is segmented into allergic conjunctivitis, postoperative inflammation, uveitis, and dry eye syndrome. The postoperative inflammation segment dominated the market with a revenue share of 48.7% in 2024, driven by the rising number of ophthalmic surgeries globally, including cataract and corneal procedures. The use of Ocular Fluorometholone is critical in reducing postoperative complications and ensuring optimal recovery. Its potent anti-inflammatory properties and physician preference for post-surgical care reinforce its dominant position. Hospitals and specialized eye clinics predominantly use this segment. Moreover, clinical guidelines and protocols often recommend 0.1% drops for postoperative inflammation. The segment also benefits from awareness campaigns by ophthalmic societies highlighting post-surgery care.
The allergic conjunctivitis segment is expected to witness the fastest CAGR of 19.2% from 2025 to 2032, driven by rising environmental allergens, increasing prevalence of seasonal and chronic eye allergies, and growing patient awareness. Physicians are increasingly prescribing corticosteroid drops for acute flare-ups when conventional antihistamines are insufficient. Convenience of administration and rapid relief of itching and redness enhance patient adherence. The segment is gaining traction in both outpatient clinics and retail pharmacy channels. Expanding e-pharmacy availability makes it easier for patients to access treatment. The development of preservative-free and combination formulations further drives growth in this subsegment.
• By Distribution Channel
On the basis of distribution channel, the ocular fluorometholone market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. The hospital pharmacies segment dominated the market with a revenue share of 53.4% in 2024, owing to direct access for patients during hospital visits and surgical procedures. Hospitals provide controlled dispensing and supervision, ensuring safe and effective corticosteroid use. Physician prescriptions and treatment protocols favor hospital pharmacies for post-operative and severe ocular conditions. The segment benefits from strong hospital networks and integration with clinical ophthalmology services. Accessibility, trust, and regulatory compliance further reinforce dominance.
The online pharmacies segment is expected to witness the fastest CAGR of 21.6% from 2025 to 2032, driven by growing patient preference for convenient home delivery and easy access to prescription medications. E-pharmacy platforms are expanding in urban and semi-urban areas, offering timely delivery of Ocular Fluorometholone. Patients increasingly value privacy, convenience, and consistent medication availability. Online pharmacies also provide opportunities for subscription-based services and refill reminders. The growth is fueled by rising digital literacy and trust in telemedicine. In addition, pharmaceutical companies are promoting online channels to enhance accessibility for chronic patients.
Ocular Fluorometholone Market Regional Analysis
- North America dominated the ocular fluorometholone market with the largest revenue share of 41.2% in 2024, driven by advanced healthcare infrastructure, early adoption of innovative ophthalmic therapies, high patient awareness, and the strong presence of key pharmaceutical companies
- The substantial growth in ocular fluorometholone usage across hospitals and clinics, supported by ongoing clinical research and post-operative treatment protocols
- The region’s well-established healthcare systems, coupled with widespread availability of specialized ophthalmologists and advanced diagnostic facilities, further reinforce market leadership
U.S. Ocular Fluorometholone Market Insight
The U.S. ocular fluorometholone market captured the largest revenue share within North America in 2024, fueled by the adoption of innovative treatment regimens and widespread clinical awareness. Increasing patient preference for minimally invasive ophthalmic therapies and postoperative anti-inflammatory treatments is driving demand. Furthermore, ongoing collaborations between pharmaceutical companies and healthcare providers to enhance patient access and affordability are contributing to the market’s growth.
Europe Ocular Fluorometholone Market Insight
The Europe ocular fluorometholone market is projected to expand at a notable CAGR during the forecast period, supported by increasing patient awareness about ophthalmic conditions and stringent regulatory standards for post-operative care. Adoption of advanced therapies and the availability of well-established hospital networks across countries such as Germany, France, and Italy are driving regional growth. In addition, European healthcare providers are increasingly emphasizing preventive eye care, boosting ocular fluorometholone prescriptions.
U.K. Ocular Fluorometholone Market Insight
The U.K. ocular fluorometholone market is expected to grow steadily during the forecast period due to rising incidences of ocular inflammation and postoperative eye treatments. The country’s strong healthcare infrastructure, combined with a focus on advanced ophthalmic care and access to innovative therapies, supports market expansion. Furthermore, growing patient awareness about potential side effects of untreated ocular inflammation encourages the adoption of fluorometholone therapies.
Germany Ocular Fluorometholone Market Insight
The Germany ocular fluorometholone market is anticipated to expand at a significant CAGR, driven by high adoption of technologically advanced ophthalmic therapies and well-developed hospital and clinic infrastructure. The presence of leading pharmaceutical companies and continuous research in ophthalmology further fuels growth. In addition, German patients increasingly prefer early treatment for post-surgical inflammation, enhancing the demand for ocular fluorometholone.
Asia-Pacific Ocular Fluorometholone Market Insight
The Asia-Pacific ocular fluorometholone market is poised to grow at the fastest CAGR of 23.5% during the forecast period of 2025–2032, driven by increasing urbanization, rising disposable incomes, expanding healthcare infrastructure, and growing access to specialized eye care in countries such as China, India, and Japan. Government initiatives to improve eye care facilities, increasing number of ophthalmology centers, and rising awareness about postoperative treatments are key growth factors in the region.
Japan Ocular Fluorometholone Market Insight
The Japan ocular fluorometholone market is gaining traction due to the country’s advanced healthcare system, aging population, and high patient awareness of postoperative eye care. Increasing prevalence of ocular inflammation and demand for effective anti-inflammatory therapies is driving adoption. Hospitals and specialized clinics are increasingly recommending fluorometholone for post-surgical management and chronic ocular conditions, enhancing market growth.
China Ocular Fluorometholone Market Insight
The China ocular fluorometholone market accounted for the largest revenue share in Asia-Pacific in 2024, fueled by rapid urbanization, expanding healthcare infrastructure, increasing patient awareness about ophthalmic therapies, and growing availability of specialized eye care centers. Rising prevalence of ocular disorders, coupled with improved access to post-surgical treatments and innovative therapies, is significantly contributing to market expansion.
Ocular Fluorometholone Market Share
The ocular fluorometholone industry is primarily led by well-established companies, including:
- Alcon Inc. (U.S.)
- Novartis AG (Switzerland)
- Santen Pharmaceutical Co., Ltd. (Japan)
- Bausch + Lomb, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Fera Pharmaceuticals (U.S.)
- Akorn, Inc. (U.S.)
- Glenmark Pharmaceuticals Ltd. (India)
- Dr. Reddy’s Laboratories Ltd. (India)
- Laboratoires Théa (France)
- Inspire Pharmaceuticals, Inc. (U.S.)
Latest Developments in Global Ocular Fluorometholone Market
- In January 2024, Amneal Pharmaceuticals launched its generic fluorometholone ophthalmic suspension, 0.1%, following approval from the U.S. FDA. The product received 180-day competitive generic therapy exclusivity, positioning it as a significant addition to the market. This launch expanded treatment options for patients with ocular inflammation and increased competition among pharmaceutical companies in the ophthalmic segment
- In April 2024, AbbVie discontinued the FML ophthalmic ointment. This discontinuation affected the availability of the product in the market, prompting healthcare providers and pharmacies to consider alternative steroidal eye treatments for patients requiring anti-inflammatory therapy
- In June 2025, the U.S. FDA approved a new sterile, topical anti-inflammatory agent for the treatment of steroid-responsive ocular inflammation. This approval represents a key advancement in ophthalmic care, offering physicians and patients an additional therapeutic option to manage inflammation and associated ocular conditions effectively
- In July 2025, Harrow announced the acquisition of certain U.S. and Canadian commercial rights for six branded ophthalmic products from Santen Pharmaceutical Co., Ltd. This strategic move strengthened Harrow’s portfolio in the ophthalmic market, enhancing its ability to provide a broader range of treatments for eye disorders, including ocular inflammation
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.