Global Oncology Biosimilars Market
Market Size in USD Billion
CAGR :
% 
 USD
6.66 Billion
USD
25.88 Billion
2024
2032
USD
6.66 Billion
USD
25.88 Billion
2024
2032
| 2025 –2032 | |
| USD 6.66 Billion | |
| USD 25.88 Billion | |
|
|
|
Oncology Biosimilars Market Analysis
A biosimilar is a biological product that closely mimics and is identical to a previously approved reference product. These are less expensive than branded or certified items. Several oncology biologics have already lost their patents, and others are about to expire. Biosimilars are predicted to gain popularity as more products lose their patents. Leading generic companies including Allergan Plc., Teva Pharmaceutical Industries Ltd., Mylan N.V., and Sandoz (a Novartis International AG division) are likely to benefit from these patent expirations and position themselves as market leaders in oncology biosimilars.
Oncology Biosimilars Market Size
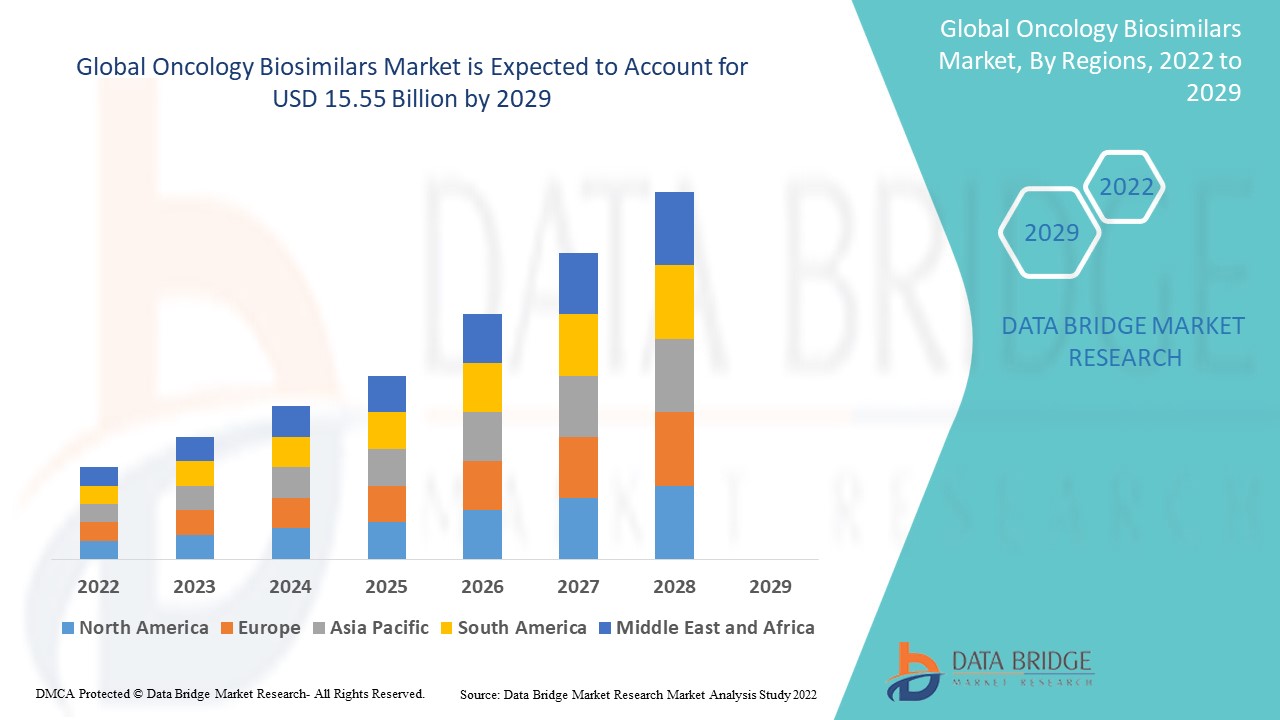
Global oncology biosimilars market size was valued at USD 6.66 billion in 2024 and is projected to reach USD 25.88 billion by 2032, with a CAGR of 18.5% during the forecast period of 2025 to 2032.
Report Scope and Market Segmentation
|
Attributes |
Oncology Biosimilars Key Market Insights |
|
Segmentation |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Pfizer Inc. (U.S.), GlaxoSmithKline plc (U.K.), Novartis AG (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd.(Israel), Sanofi (France), F. Hoffmann-La Roche Ltd. (Switzerland), Zydus Cadila (India), Lupin (India), Amneal Pharmaceuticals LLC. (U.S.), Cipla Inc. (U.S.), Aurobindo Pharma (India), Glenmark Pharmaceuticals Limited (India), Eli Lilly and Company (U.S.), Sun Pharmaceutical Industries Ltd. (India), Allergan (Ireland), Bristol-Myers Squibb Company (U.S.), Takeda Pharmaceutical Company Limited (Japan), BIOCAD (Russia), Apotex Inc. (Canada), Endo International plc (Ireland) |
|
Market Opportunities |
|
Oncology Biosimilars Market Definition
A biosimilar is a generic counterpart of a well-known biologic medicine. Medications made from living substances such as cells, tissues, or proteins are known as biologic drugs. A biosimilar is a medication that is based on a biologic drug that has already been studied, produced, clinically tested for safety and effectiveness, and authorized by the US Food and Drug Administration (FDA). Since the biosimilar is based on the approved drug, the biologic medications are commonly referred to as reference drugs. The functions of biosimilar are same as the reference drugs and it is dependent to FDA approval.
Oncology Biosimilars Market Dynamics
Drivers
- Rise in the prevalence of cancer
The surging prevalence of cancer is a major factor driving the oncology biosimilars market's growth rate during the forecast period of 2025-2032. Cancer is a lifestyle disease caused by abnormal cell proliferation that can lead to the formation of a tumor. Biological medications, often known as biologics, are used in the majority of effective cancer treatments nowadays, including targeted therapies and immunotherapies. These medications are derived from living creatures such as bacteria, animal or plant cells, yeast and they require complicated manufacturing techniques and a long development time. The development of biosimilars of branded cancer biologics is gaining popularity as a way to reduce treatment costs.
- Growing number of government initiatives
The rising initiatives by government is expected to expand the oncology biosimilars market. Additionally, Biosimilars are being promoted by governments in a number of nations as a cost-cutting solution. The United States Food and Drug Administration (USFDA), for instance, has created educational materials for physicians and patients on biosimilars.
Furthermore, sedentary lifestyle of people and surging geriatric population will result in the expansion of oncology biosimilars market. Along with this, rising healthcare expenditure and increasing demand for biosimilar drugs due to their cost-effectiveness will enhance the growth rate of the market.
Opportunities
- Increase in the number of research and development activities
The rising number of research and development activities for new indications and patent expiry of biologics is estimated to create new opportunities for the oncology biosimilars market growth in coming years. The players are investing in these research activities in order to test different formulas and increase revenue. The competitors also engage in strategic alliances, which aid in significantly affecting the oncology biosimilars market's growth rate.
Moreover, rising investment for the development of advanced technologies and increase in the number of emerging markets will further provide beneficial opportunities for the oncology biosimilars market growth during the forecast period.
Restraints/Challenges
On the other hand, high cost associated with the drug development and distribution will obstruct the growth rate of market. The lack of healthcare infrastructure in developing economies and dearth of skilled professionals will challenge the oncology biosimilars market. Additionally, regulatory as well as clinical barriers and resistance from biologics manufacturers will act as restrain and further impede the growth rate of market during the forecast period of 2025-2032.
This oncology biosimilars market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the oncology biosimilars market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Patient Epidemiology Analysis
Oncology biosimilars market also provides you with detailed market analysis for patient analysis, prognosis and cures. Prevalence, incidence, mortality, adherence rates are some of the data variables that are available in the report. Direct or indirect impact analyses of epidemiology to market growth are analysed to create a more robust and cohort multivariate statistical model for forecasting the market in the growth period.
Oncology Biosimilars Market Scope
The oncology biosimilars market is segmented on the basis of indication, drug class, route of administration, end-users and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Indication
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Cervical Cancer
- Blood Cancer
- Others
Drug Class
- Monoclonal Antibodies
- Granulocyte Colony-Stimulating Factor
- Others
Route of Administration
- Intravenous
- Subcutaneous
- Others
End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Oncology Biosimilars Market Regional Analysis
The oncology biosimilars market is analyzed and market size insights and trends are provided by country, indication, drug class, route of administration, end-users and distribution channel as referenced above.
The countries covered in the oncology biosimilars market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
North America dominates the oncology biosimilars market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the presence of major key players and rising healthcare expenditure will further propel the market’s growth rate in this region. Additionally, surging prevalence of cancer will further propel the market’s growth rate in this region.
Asia-Pacific is expected to grow during the forecast period of 2025-2032 due to large number of generic manufacturer in this region. Also, development of healthcare infrastructure and rising geriatric population will further propel the market’s growth rate in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Oncology Biosimilars Market Share
The oncology biosimilars market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to oncology biosimilars market.
Oncology Biosimilars Market Leaders Operating in the Market Are:
- Pfizer Inc. (U.S.)
- GlaxoSmithKline plc (U.K.)
- Novartis AG (Switzerland)
- Mylan N.V. (U.S.)
- Teva Pharmaceutical Industries Ltd.(Israel)
- Sanofi (France)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Zydus Cadila (India)
- Lupin (India)
- Amneal Pharmaceuticals LLC. (U.S.)
- Cipla Inc. (U.S.)
- Aurobindo Pharma (India)
- Glenmark Pharmaceuticals Limited (India)
- Eli Lilly and Company (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Allergan (Ireland)
- Bristol-Myers Squibb Company (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- BIOCAD (Russia)
- Apotex Inc. (Canada)
- Endo International plc (Ireland)
Latest Developments in Oncology Biosimilars Market
- In December 2020, Amgen had received United States Food and Drug Administration (USFDA) approval of RIABNI (rituximab-arrx). RIABNI (rituximab-arrx) is a biosimilar to rituximab which is used for the treatment of adult patients suffering from non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, Wegener's granulomatosis and microscopic polyangiitis (MPA)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.