Global Oncology Molecular Diagnostic Market
Market Size in USD Billion
CAGR :
% 
 USD
3.69 Billion
USD
11.45 Billion
2022
2030
USD
3.69 Billion
USD
11.45 Billion
2022
2030
| 2023 –2030 | |
| USD 3.69 Billion | |
| USD 11.45 Billion | |
|
|
|
|
Oncology Molecular Diagnostic Market Analysis and Size
According to the American Cancer Society's 2020 report, several professional organizations, including the National Comprehensive Cancer Network (NCCN), the American Association of Clinical Oncology (ASCO), and the College of American Pathologists (CAP), have developed biomarker testing and treatment guidelines. Furthermore, the rising cancer prevalence in the country contributes to the region's oncology molecular diagnostics market. According to the National Cancer Institute's (NCI) 2020 Statistics, approximately 1,806,590 cases were diagnosed in the United States in 2020, with the number of cancer survivors expected to reach 2.2 million by 2030. Furthermore, according to the NCI, breast cancer will be the most common cancer type in 2021, accounting for 284,200 cases. These factors are causing an increase in demand for oncology molecular diagnostic market.
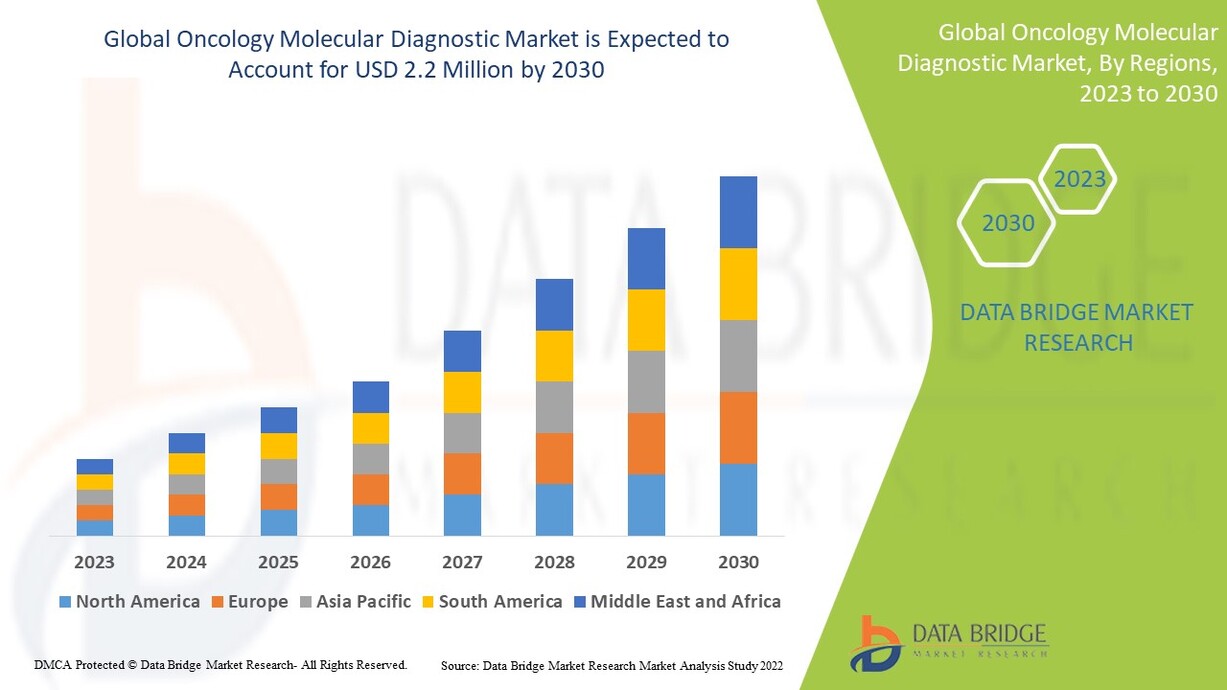
Data Bridge Market Research analyses that the oncology molecular diagnostic market which was USD 3.69 billion in 2022, is expected to reach USD 11.45 billion by 2030, at a CAGR of 15.2% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Oncology Molecular Diagnostic Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Breast Cancer, Prostate Cancer, Colorectal Cancer, Cervical Cancer, Liver Cancer, Lung Cancer, Blood Cancer, Kidney Cancer, Others), Product (Instruments, Reagents, Others), Test Location (Clinics and Other Establishments, Point of Care), Technology (PCR, In Situ Hybridization, Isothermal Nucleic Acid Amplification Technology (INAAT), Chips and Microarrays, Mass Spectrometry, Sequencing, Transcription Mediated Amplification, Others), End User (Hospitals, Diagnostic Centers) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Abbott (U.S.), Bayer AG (Germany), BD (U.S.), Agilent Technologies, Inc (U.S.), Danaher (U.S.), Hologic, Inc. (U.S.), QIAGEN (Germany), F. Hoffmann-La Roche Ltd (Switzerland), Siemens Healthcare Private Limited (Germany), Sysmex Singapore Pte. Ltd. (Singapore), BIOMÉRIEUX (France), DiaSorin Molecular LLC (U.S.), Grifols, S.A. (Spain), Illumina, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), Leica Biosystems Nussloch GmbH (Germany), Analytik Jena GmbH (Germany), Biocartis (Belgium), GenMark Diagnostics, Inc (U.S.), HTG Molecular Diagnostics, Inc. (U.S.) |
|
Market Opportunities |
|
Market Definition
Oncology molecular diagnostics detect cancer cells by examining their biological molecules. Several tests on blood, saliva, and tumour tissue samples are performed to detect and measure specific genetic sequences in DNA, RNA, and cell proteins. Oncology molecular diagnostics aid in the rapid analysis and provision of detailed information, which is then used in the personalized treatment of cancer. These diagnostics solutions are used in clinical and point-of-care (POC) testing for cancer detection. Blood banks are also used to identify pathogens and infectious diseases in donated blood samples.
Oncology Molecular Diagnostic Market Dynamics
Drivers
- Rising prevalence of cancer cases
The rising prevalence of cancer cases around the world is driving up demand for diagnosis, which is driving up the oncology molecular diagnostics market. Cancer was responsible for approximately 10 million deaths in 2020, according to the World Health Organization (WHO). Furthermore, according to the American Cancer Society's 2021 statistics, the global burden of cancer is expected to rise to 27.5 million new cases and 16.3 million cancer deaths by 2040. Such high figures suggest that the estimated rising prevalence of cancer is contributing to the growing need for early detection and preventive medicine. As a result of the abovementioned factors, the demand for oncology molecular diagnostic is steadily increasing.
Opportunities
- New advancements in molecular diagnostics tests
As diagnostic testing technology advances, molecular diagnostics tests for oncology are becoming more popular. Several passages are being made in cancer genome sequencing, computational analysis, tumour models, and innovative cancer research methods. For instance, Roche Molecular Systems, Inc. developed the Cobas EGFR Mutation Test for non-small cell lung cancer patients, which received FDA approval in 2020 and is a real-time PCR test that detects 42 mutations in the epidermal growth factor receptor (EGFR) gene.
Restraints/Challenges
- High cost of molecular diagnostic tests
The high cost of molecular diagnostic tests is a major impediment to the growth of the oncology molecular diagnostic market. The cost of molecular diagnostic testing in cancer care is comparable higher, and it varies depending on the type of therapy. For instance, a molecular diagnostic test for non-small cell lung cancer monotherapy costs around $11,000 to $20,000 per month, and these prices are expected to rise with combination therapy. As a result, the high cost of molecular diagnostic tests is expected to hamper the market for oncology molecular diagnostic.
This oncology molecular diagnostic market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the oncology molecular diagnostic market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on the Oncology Molecular Diagnostic Market
The COVID-19 pandemic has significantly impacted the oncology molecular diagnostic market. According to 85 percent of respondents in a 2021 study of 164 laboratories conducted by the Association for Molecular Pathology (AMP), molecular testing for cancer decreased between April and June 2020. Furthermore, more than half of respondents stated that oncology testing for clinical trials has reduced due to lower enrolment, aversion to travel, and testing capacity. According to the survey results, the pandemic may have long-term implications for cancer molecular diagnostic testing. Furthermore, cancer research and development institutes and universities worldwide have ceased operations. Furthermore, government funding for research from a variety of institutions is on hold in several parts of the country.
Recent Developments
- In 2021, Amoy Diagnostics Co. Ltd, Riken Genesis Co. Ltd, and Precision Medicine Asia Co. Ltd launched the AmoyDx Pan Lung Cancer PCR Panel. It has been approved for marketing and production in Japan by the Ministry of Health, Labour, and Welfare (MHLW).
- In 2020, Veracyte Inc. and Bayer AG collaborated to advance the Precision Oncology Patient Identification Program in thyroid cancer. The advancement will allow testing with Veracyte's Afirma Xpression Atlas (XA) to identify underlying genomic drivers in tumour cells, and the biomarker-driven therapies may be limited to patients with advanced thyroid cancer.
- In 2020, Danaher (Cepheid) announced a collaboration with Sherlock Biosciences to investigate the development of innovative, ground-breaking molecular diagnostic tests for oncology and infectious diseases using CRISPR technology.
Global Oncology Molecular Diagnostic Market Scope
The oncology molecular diagnostic market is segmented on the basis of type, product, test location, technology, and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Breast Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Liver Cancer
- Lung Cancer
- Blood Cancer
- Kidney Cancer
- Others
Product
- Instruments
- Reagents
- Others
Test Location
- Clinics
- Other Establishments
- Point of Care
Technology
- PCR
- Multiplex
- Others
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
End User
- Hospitals
- Diagnostic Centers
Oncology Molecular Diagnostic Market Regional Analysis/Insights
The oncology molecular diagnostic market is analyzed and market size insights and trends are provided by country, type, product, test location, technology, and end user as referenced above.
The countries covered in the oncology molecular diagnostic market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the oncology molecular diagnostic market because of the prevalence of well-established healthcare infrastructure and the growing number of healthcare infrastructure.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 due to increasing health conscious people, as a result of increasing cancer occurrences and changes in lifestyle associated with economic development in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The oncology molecular diagnostic market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for oncology molecular diagnostic market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the oncology molecular diagnostic market. The data is available for historic period 2011-2021.
Competitive Landscape and Oncology Molecular Diagnostic Market Share Analysis
The oncology molecular diagnostic market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to oncology molecular diagnostic market.
Some of the major players operating in the oncology molecular diagnostic market are:
- Abbott (U.S.)
- Bayer AG (Germany)
- BD (U.S.)
- Agilent Technologies, Inc (U.S.)
- Danaher (U.S.)
- Hologic, Inc. (U.S.)
- QIAGEN (Germany)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Siemens Healthcare Private Limited (Germany)
- Sysmex Singapore Pte. Ltd. (Singapore)
- BIOMÉRIEUX (France)
- DiaSorin Molecular LLC (U.S.)
- Grifols, S.A. (Spain)
- Illumina, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Leica Biosystems Nussloch GmbH (Germany)
- Analytik Jena GmbH (Germany)
- Biocartis (Belgium)
- GenMark Diagnostics, Inc (U.S.)
- HTG Molecular Diagnostics, Inc. (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKETSIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 TEST DATA VOLUME
2.2.12 EPIDEMIOLOGY
2.2.13 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.14 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 KEY TRENDS
5.2 PRODUCT INNOVATION & TECHNOLOGY OVERVIEW
5.3 KEY PLAYERS STRATEGIES
5.4 LONG TERM GROWTH APPROACH
5.5 OTHERS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 DIAGNOSIS RATE
6.3 MORTALITY RATE
6.4 PATEINT TREATMENT SUCCESS RATES
7 INDUSTRY INSIGHTS
7.1 PATENT ANALYSIS
7.2 ONCOLOGY MOLECULAR DIAGNOSTIC RATE BY MATURED MARKETS
7.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
7.4 PATIENT FLOW DIAGRAM
7.5 KEY PRICING STRATEGIES
7.6 KEY PATIENT ENROLLMENT STRATEGIES
7.7 INTERVIEWS WITH VIROLOGIST
7.8 OTHER KOL SNAPSHOTS
8 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, BY PRODUCT
8.1 OVERVIEW
8.2 KITS & REAGENTS
8.2.1 MUTATIONS DETECTION KIT
8.2.1.1. MARKET VALUE (USD MILLION)
8.2.1.2. MARKET VOLUME (UNITS)
8.2.1.3. AVERAGE SELLING PRICE (USD)
8.2.2 PANEL KIT
8.2.2.1. MARKET VALUE (USD MILLION)
8.2.2.2. MARKET VOLUME (UNITS)
8.2.2.3. AVERAGE SELLING PRICE (USD)
8.2.3 MPN PANEL KIT
8.2.3.1. MARKET VALUE (USD MILLION)
8.2.3.2. MARKET VOLUME (UNITS)
8.2.3.3. AVERAGE SELLING PRICE (USD)
8.2.4 AML PANEL KIT
8.2.4.1. MARKET VALUE (USD MILLION)
8.2.4.2. MARKET VOLUME (UNITS)
8.2.4.3. AVERAGE SELLING PRICE (USD)
8.2.5 KRAS PCR KIT
8.2.5.1. MARKET VALUE (USD MILLION)
8.2.5.2. MARKET VOLUME (UNITS)
8.2.5.3. AVERAGE SELLING PRICE (USD)
8.2.6 EGFR KIT
8.2.6.1. MARKET VALUE (USD MILLION)
8.2.6.2. MARKET VOLUME (UNITS)
8.2.6.3. AVERAGE SELLING PRICE (USD)
8.2.7 BRAF MUTATION KIT
8.2.7.1. MARKET VALUE (USD MILLION)
8.2.7.2. MARKET VOLUME (UNITS)
8.2.7.3. AVERAGE SELLING PRICE (USD)
8.2.8 ALL PANEL KIT
8.2.8.1. MARKET VALUE (USD MILLION)
8.2.8.2. MARKET VOLUME (UNITS)
8.2.8.3. AVERAGE SELLING PRICE (USD)
8.2.9 AML1-ETO KIT
8.2.9.1. MARKET VALUE (USD MILLION)
8.2.9.2. MARKET VOLUME (UNITS)
8.2.9.3. AVERAGE SELLING PRICE (USD)
8.2.10 NRAS MUTATION KIT
8.2.10.1. MARKET VALUE (USD MILLION)
8.2.10.2. MARKET VOLUME (UNITS)
8.2.10.3. AVERAGE SELLING PRICE (USD)
8.2.11 CALR KIT
8.2.11.1. MARKET VALUE (USD MILLION)
8.2.11.2. MARKET VOLUME (UNITS)
8.2.11.3. AVERAGE SELLING PRICE (USD)
8.2.12 FLT3 MUTATION DETECTION
8.2.12.1. MARKET VALUE (USD MILLION)
8.2.12.2. MARKET VOLUME (UNITS)
8.2.12.3. AVERAGE SELLING PRICE (USD)
8.2.13 C-KIT MUTATION DETECTION KIT
8.2.13.1. MARKET VALUE (USD MILLION)
8.2.13.2. MARKET VOLUME (UNITS)
8.2.13.3. AVERAGE SELLING PRICE (USD)
8.2.14 MGMT METHYLATION DETECTION KIT
8.2.14.1. MARKET VALUE (USD MILLION)
8.2.14.2. MARKET VOLUME (UNITS)
8.2.14.3. AVERAGE SELLING PRICE (USD)
8.2.15 CBFB-MYH11 KIT
8.2.15.1. MARKET VALUE (USD MILLION)
8.2.15.2. MARKET VOLUME (UNITS)
8.2.15.3. AVERAGE SELLING PRICE (USD)
8.2.16 GENE FUSIONS DETECTION KIT
8.2.16.1. MARKET VALUE (USD MILLION)
8.2.16.2. MARKET VOLUME (UNITS)
8.2.16.3. AVERAGE SELLING PRICE (USD)
8.2.17 OTHER
8.3 INSTRUMENTS
8.3.1 BY TYPE
8.3.1.1. PCR SYSTEM
8.3.1.1.1. MARKET VALUE (USD MILLION)
8.3.1.1.2. MARKET VOLUME (UNITS)
8.3.1.1.3. AVERAGE SELLING PRICE (USD)
8.3.1.2. ANALYZER
8.3.1.2.1. MARKET VALUE (USD MILLION)
8.3.1.2.2. MARKET VOLUME (UNITS)
8.3.1.2.3. AVERAGE SELLING PRICE (USD)
8.3.1.3. MICROARRAY SCANNER
8.3.1.3.1. MARKET VALUE (USD MILLION)
8.3.1.3.2. MARKET VOLUME (UNITS)
8.3.1.3.3. AVERAGE SELLING PRICE (USD)
8.3.1.4. GENETIC ANALYSIS SYSTEM
8.3.1.4.1. MARKET VALUE (USD MILLION)
8.3.1.4.2. MARKET VOLUME (UNITS)
8.3.1.4.3. AVERAGE SELLING PRICE (USD)
8.3.1.5. MASS SPECTROMETER
8.3.1.5.1. MARKET VALUE (USD MILLION)
8.3.1.5.2. MARKET VOLUME (UNITS)
8.3.1.5.3. AVERAGE SELLING PRICE (USD)
8.3.1.6. THERMOSHAKERS
8.3.1.6.1. MARKET VALUE (USD MILLION)
8.3.1.6.2. MARKET VOLUME (UNITS)
8.3.1.6.3. AVERAGE SELLING PRICE (USD)
8.3.1.7. HIGH THROUGHPUT SYSTEMS
8.3.1.7.1. MARKET VALUE (USD MILLION)
8.3.1.7.2. MARKET VOLUME (UNITS)
8.3.1.7.3. AVERAGE SELLING PRICE (USD)
8.3.1.8. THERMAL CYCLER
8.3.1.8.1. MARKET VALUE (USD MILLION)
8.3.1.8.2. MARKET VOLUME (UNITS)
8.3.1.8.3. AVERAGE SELLING PRICE (USD)
8.3.1.9. POINT OF CARE DEVICES
8.3.1.9.1. MARKET VALUE (USD MILLION)
8.3.1.9.2. MARKET VOLUME (UNITS)
8.3.1.9.3. AVERAGE SELLING PRICE (USD)
8.3.1.10. PLATFORM/SYSTEM
8.3.1.10.1. MARKET VALUE (USD MILLION)
8.3.1.10.2. MARKET VOLUME (UNITS)
8.3.1.10.3. AVERAGE SELLING PRICE (USD)
8.3.1.11. OTHERS
8.3.2 BY MODALITY
8.3.2.1. STANDALONE
8.3.2.2. PORTABLE
8.3.2.3. BENCHTOP
8.3.3 BY USABILITY
8.3.3.1. FULLY AUTOMATED INSTRUMENTS
8.3.3.2. SEMI-AUTOMATED INSTRUMENTS
9 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, BY TECHNOLOGY
9.1 OVERVIEW
9.2 POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
9.2.1 REAL-TIME PCR
9.2.2 DIGITAL PCR
9.2.3 REVERSE TRANSCRIPTASE PCR
9.2.4 QUANTITATIVE FLUORESCENT PCR
9.2.5 OTHER
9.3 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
9.4 TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
9.5 SEQUENCING
9.5.1 NEXT GENERATION SEQUENCING (NGS)
9.5.2 FIRST GENERATION SEQUENCING
9.5.3 SECOND GENERATION SEQUENCING
9.5.4 THIRD GENERATION SEQUENCING
9.6 MASS SPECTROMETRY (MS)
9.7 CHIPS AND MICROARRAY
9.8 CAPILLARY ELECTROPHORESIS
9.9 IN SITU HYBRIDIZATION (ISH OR FISH)
9.1 OTHERS
10 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, BY CANCER TYPE
10.1 OVERVIEW
10.2 BREAST CANCER
10.2.1 BY PRODUCT
10.2.1.1. KITS & REAGENTS
10.2.1.2. INSTRUMENTS
10.2.2 BY MODE
10.2.2.1. LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
10.2.2.2. POINT-OF-CARE
10.2.3 BY TECHNOLOGY
10.2.3.1. POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
10.2.3.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
10.2.3.3. TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
10.2.3.4. SEQUENCING
10.2.3.5. MASS SPECTROMETRY (MS)
10.2.3.6. CHIPS AND MICROARRAY
10.2.3.7. CAPILLARY ELECTROPHORESIS
10.2.3.8. IN SITU HYBRIDIZATION (ISH OR FISH)
10.2.3.9. OTHERS
10.3 PROSTATE CANCER
10.3.1 BY PRODUCT
10.3.1.1. KITS & REAGENTS
10.3.1.2. INSTRUMENTS
10.3.2 BY MODE
10.3.2.1. LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
10.3.2.2. POINT-OF-CARE
10.3.3 BY TECHNOLOGY
10.3.3.1. POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
10.3.3.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
10.3.3.3. TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
10.3.3.4. SEQUENCING
10.3.3.5. MASS SPECTROMETRY (MS)
10.3.3.6. CHIPS AND MICROARRAY
10.3.3.7. CAPILLARY ELECTROPHORESIS
10.3.3.8. IN SITU HYBRIDIZATION (ISH OR FISH)
10.3.3.9. OTHERS
10.4 COLORECTAL CANCER
10.4.1 BY PRODUCT
10.4.1.1. KITS & REAGENTS
10.4.1.2. INSTRUMENTS
10.4.2 BY MODE
10.4.2.1. LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
10.4.2.2. POINT-OF-CARE
10.4.3 BY TECHNOLOGY
10.4.3.1. POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
10.4.3.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
10.4.3.3. TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
10.4.3.4. SEQUENCING
10.4.3.5. MASS SPECTROMETRY (MS)
10.4.3.6. CHIPS AND MICROARRAY
10.4.3.7. CAPILLARY ELECTROPHORESIS
10.4.3.8. IN SITU HYBRIDIZATION (ISH OR FISH)
10.4.3.9. OTHERS
10.5 CERVICAL CANCER
10.5.1 BY PRODUCT
10.5.1.1. KITS & REAGENTS
10.5.1.2. INSTRUMENTS
10.5.2 BY MODE
10.5.2.1. LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
10.5.2.2. POINT-OF-CARE
10.5.3 BY TECHNOLOGY
10.5.3.1. POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
10.5.3.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
10.5.3.3. TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
10.5.3.4. SEQUENCING
10.5.3.5. MASS SPECTROMETRY (MS)
10.5.3.6. CHIPS AND MICROARRAY
10.5.3.7. CAPILLARY ELECTROPHORESIS
10.5.3.8. IN SITU HYBRIDIZATION (ISH OR FISH)
10.5.3.9. OTHERS
10.6 LIVER CANCER
10.6.1 BY PRODUCT
10.6.1.1. KITS & REAGENTS
10.6.1.2. INSTRUMENTS
10.6.2 BY MODE
10.6.2.1. LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
10.6.2.2. POINT-OF-CARE
10.6.3 BY TECHNOLOGY
10.6.3.1. POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
10.6.3.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
10.6.3.3. TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
10.6.3.4. SEQUENCING
10.6.3.5. MASS SPECTROMETRY (MS)
10.6.3.6. CHIPS AND MICROARRAY
10.6.3.7. CAPILLARY ELECTROPHORESIS
10.6.3.8. IN SITU HYBRIDIZATION (ISH OR FISH)
10.6.3.9. OTHERS
10.7 LUNG CANCER
10.7.1 BY PRODUCT
10.7.1.1. KITS & REAGENTS
10.7.1.2. INSTRUMENTS
10.7.2 BY MODE
10.7.2.1. LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
10.7.2.2. POINT-OF-CARE
10.7.3 BY TECHNOLOGY
10.7.3.1. POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
10.7.3.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
10.7.3.3. TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
10.7.3.4. SEQUENCING
10.7.3.5. MASS SPECTROMETRY (MS)
10.7.3.6. CHIPS AND MICROARRAY
10.7.3.7. CAPILLARY ELECTROPHORESIS
10.7.3.8. IN SITU HYBRIDIZATION (ISH OR FISH)
10.7.3.9. OTHERS
10.8 BLOOD CANCER
10.8.1 BY PRODUCT
10.8.1.1. KITS & REAGENTS
10.8.1.2. INSTRUMENTS
10.8.2 BY MODE
10.8.2.1. LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
10.8.2.2. POINT-OF-CARE
10.8.3 BY TECHNOLOGY
10.8.3.1. POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
10.8.3.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
10.8.3.3. TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
10.8.3.4. SEQUENCING
10.8.3.5. MASS SPECTROMETRY (MS)
10.8.3.6. CHIPS AND MICROARRAY
10.8.3.7. CAPILLARY ELECTROPHORESIS
10.8.3.8. IN SITU HYBRIDIZATION (ISH OR FISH)
10.8.3.9. OTHERS
10.9 KIDNEY CANCER
10.9.1 BY PRODUCT
10.9.1.1. KITS & REAGENTS
10.9.1.2. INSTRUMENTS
10.9.2 BY MODE
10.9.2.1. LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
10.9.2.2. POINT-OF-CARE
10.9.3 BY TECHNOLOGY
10.9.3.1. POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
10.9.3.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
10.9.3.3. TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
10.9.3.4. SEQUENCING
10.9.3.5. MASS SPECTROMETRY (MS)
10.9.3.6. CHIPS AND MICROARRAY
10.9.3.7. CAPILLARY ELECTROPHORESIS
10.9.3.8. IN SITU HYBRIDIZATION (ISH OR FISH)
10.9.3.9. OTHERS
10.1 MELANOMA
10.10.1 BY PRODUCT
10.10.1.1. KITS & REAGENTS
10.10.1.2. INSTRUMENTS
10.10.2 BY MODE
10.10.2.1. LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
10.10.2.2. POINT-OF-CARE
10.10.3 BY TECHNOLOGY
10.10.3.1. POLYMERASE CHAIN REACTION (PCR)-BASED METHODS
10.10.3.2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT)
10.10.3.3. TRANSCRIPTION MEDIATED AMPLIFICATION (TMA)
10.10.3.4. SEQUENCING
10.10.3.5. MASS SPECTROMETRY (MS)
10.10.3.6. CHIPS AND MICROARRAY
10.10.3.7. CAPILLARY ELECTROPHORESIS
10.10.3.8. IN SITU HYBRIDIZATION (ISH OR FISH)
10.10.3.9. OTHERS
10.11 OTHERS
11 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, BY MODE
11.1 OVERVIEW
11.2 LABORATORY/ CLINICS AND OTHER ESTABLISHMENTS
11.2.1 KITS & REAGENTS
11.2.2 INSTRUMENTS
11.3 POINT-OF-CARE
11.3.1 KITS & REAGENTS
11.3.2 INSTRUMENTS
12 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.2.1 BY TYPE
12.2.1.1. TIER 1
12.2.1.2. TIER 2
12.2.1.3. TIER 3
12.2.2 BY PRODUCT
12.2.2.1. KITS & REAGENTS
12.2.2.2. INSTRUMENTS
12.3 SPECIALITY CLINICS
12.3.1 KITS & REAGENTS
12.3.2 INSTRUMENTS
12.4 DIAGNOSTIC LABORATORIES
12.4.1 KITS & REAGENTS
12.4.2 INSTRUMENTS
12.5 CANCER RESEARCH INSTITUTES BY PRODUCT
12.5.1 KITS & REAGENTS
12.5.2 INSTRUMENTS
12.6 ACADEMIC AND RESEARCH INSTITUTES
12.6.1 KITS & REAGENTS
12.6.2 INSTRUMENTS
12.7 OTHERS
13 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDERS
13.3 RETAIL SALES
13.4 ONLINE SALES
13.5 OTHERS
14 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
14.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
14.3 COMPANY SHARE ANALYSIS: EUROPE
14.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
14.5 MERGERS & ACQUISITIONS
14.6 NEW PRODUCT DEVELOPMENT & APPROVALS
14.7 EXPANSIONS
14.8 REGULATORY CHANGES
14.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
14.1 MARKET ATTRACTIVENESS
15 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, BY REGION
GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
15.2 EUROPE
15.2.1 GERMANY
15.2.2 U.K.
15.2.3 ITALY
15.2.4 FRANCE
15.2.5 SPAIN
15.2.6 RUSSIA
15.2.7 SWITZERLAND
15.2.8 TURKEY
15.2.9 BELGIUM
15.2.10 NETHERLANDS
15.2.11 DENMARK
15.2.12 SWEDEN
15.2.13 POLAND
15.2.14 NORWAY
15.2.15 FINLAND
15.2.16 REST OF EUROPE
15.3 ASIA-PACIFIC
15.3.1 JAPAN
15.3.2 CHINA
15.3.3 SOUTH KOREA
15.3.4 INDIA
15.3.5 SINGAPORE
15.3.6 THAILAND
15.3.7 INDONESIA
15.3.8 MALAYSIA
15.3.9 PHILIPPINES
15.3.10 AUSTRALIA
15.3.11 NEW ZEALAND
15.3.12 VIETNAM
15.3.13 TAIWAN
15.3.14 REST OF ASIA-PACIFIC
15.4 LATIN AMERICA
15.4.1 BRAZIL
15.4.2 ARGENTINA
15.4.3 REST OF LATIN AMERICA
15.5 MIDDLE EAST AND AFRICA
15.5.1 SOUTH AFRICA
15.5.2 EGYPT
15.5.3 BAHRAIN
15.5.4 UNITED ARAB EMIRATES
15.5.5 KUWAIT
15.5.6 OMAN
15.5.7 QATAR
15.5.8 SAUDI ARABIA
15.5.9 REST OF MEA
15.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
16 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, SWOT ANALYSIS
17 GLOBAL ONCOLOGY MOLECULAR DIAGNOSTIC MARKET, COMPANY PROFILE
17.1 ABBOTT LABORATORIES
17.1.1 COMPANY OVERVIEW
17.1.2 REVENUE ANALYSIS
17.1.3 GEOGRAPHIC PRESENCE
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPEMENTS
17.2 F. HOFFMANN-LA ROCHE LTD
17.2.1 COMPANY OVERVIEW
17.2.2 REVENUE ANALYSIS
17.2.3 GEOGRAPHIC PRESENCE
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPEMENTS
17.3 ILLUMINA, INC.
17.3.1 COMPANY OVERVIEW
17.3.2 REVENUE ANALYSIS
17.3.3 GEOGRAPHIC PRESENCE
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPEMENTS
17.4 HOLOGIC, INC.
17.4.1 COMPANY OVERVIEW
17.4.2 REVENUE ANALYSIS
17.4.3 GEOGRAPHIC PRESENCE
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPEMENTS
17.5 BIOMÉRIEUX
17.5.1 COMPANY OVERVIEW
17.5.2 REVENUE ANALYSIS
17.5.3 GEOGRAPHIC PRESENCE
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPEMENTS
17.6 AGILENT TECHNOLOGIES, INC
17.6.1 COMPANY OVERVIEW
17.6.2 REVENUE ANALYSIS
17.6.3 GEOGRAPHIC PRESENCE
17.6.4 PRODUCT PORTFOLIO
17.6.5 RECENT DEVELOPEMENTS
17.7 BD
17.7.1 COMPANY OVERVIEW
17.7.2 REVENUE ANALYSIS
17.7.3 GEOGRAPHIC PRESENCE
17.7.4 PRODUCT PORTFOLIO
17.7.5 RECENT DEVELOPEMENTS
17.8 BECKMAN COULTER, INC.
17.8.1 COMPANY OVERVIEW
17.8.2 REVENUE ANALYSIS
17.8.3 GEOGRAPHIC PRESENCE
17.8.4 PRODUCT PORTFOLIO
17.8.5 RECENT DEVELOPEMENTS
17.9 THERMO FISHER SCIENTIFIC INC.
17.9.1 COMPANY OVERVIEW
17.9.2 REVENUE ANALYSIS
17.9.3 GEOGRAPHIC PRESENCE
17.9.4 PRODUCT PORTFOLIO
17.9.5 RECENT DEVELOPEMENTS
17.1 BIODESIX
17.10.1 COMPANY OVERVIEW
17.10.2 REVENUE ANALYSIS
17.10.3 GEOGRAPHIC PRESENCE
17.10.4 PRODUCT PORTFOLIO
17.10.5 RECENT DEVELOPEMENTS
17.11 INTEGRATED DNA TECHNOLOGIES, INC.
17.11.1 COMPANY OVERVIEW
17.11.2 REVENUE ANALYSIS
17.11.3 GEOGRAPHIC PRESENCE
17.11.4 PRODUCT PORTFOLIO
17.11.5 RECENT DEVELOPEMENTS
17.12 BIONTECH SE.
17.12.1 COMPANY OVERVIEW
17.12.2 REVENUE ANALYSIS
17.12.3 GEOGRAPHIC PRESENCE
17.12.4 PRODUCT PORTFOLIO
17.12.5 RECENT DEVELOPEMENTS
17.13 SIEMENS HEALTHCARE GMBH
17.13.1 COMPANY OVERVIEW
17.13.2 REVENUE ANALYSIS
17.13.3 GEOGRAPHIC PRESENCE
17.13.4 PRODUCT PORTFOLIO
17.13.5 RECENT DEVELOPEMENTS
17.14 BAYER AG
17.14.1 COMPANY OVERVIEW
17.14.2 REVENUE ANALYSIS
17.14.3 GEOGRAPHIC PRESENCE
17.14.4 PRODUCT PORTFOLIO
17.14.5 RECENT DEVELOPEMENTS
17.15 SYSMEX SE,
17.15.1 COMPANY OVERVIEW
17.15.2 REVENUE ANALYSIS
17.15.3 GEOGRAPHIC PRESENCE
17.15.4 PRODUCT PORTFOLIO
17.15.5 RECENT DEVELOPEMENTS
17.16 DANAHER
17.16.1 COMPANY OVERVIEW
17.16.2 REVENUE ANALYSIS
17.16.3 GEOGRAPHIC PRESENCE
17.16.4 PRODUCT PORTFOLIO
17.16.5 RECENT DEVELOPEMENTS
17.17 QIAGEN
17.17.1 COMPANY OVERVIEW
17.17.2 REVENUE ANALYSIS
17.17.3 GEOGRAPHIC PRESENCE
17.17.4 PRODUCT PORTFOLIO
17.17.5 RECENT DEVELOPEMENTS
17.18 CEPHEID
17.18.1 COMPANY OVERVIEW
17.18.2 REVENUE ANALYSIS
17.18.3 GEOGRAPHIC PRESENCE
17.18.4 PRODUCT PORTFOLIO
17.18.5 RECENT DEVELOPEMENTS
17.19 VERACYTE, INC
17.19.1 COMPANY OVERVIEW
17.19.2 REVENUE ANALYSIS
17.19.3 GEOGRAPHIC PRESENCE
17.19.4 PRODUCT PORTFOLIO
17.19.5 RECENT DEVELOPEMENTS
17.2 DIASORIN MOLECULAR LLC
17.20.1 COMPANY OVERVIEW
17.20.2 REVENUE ANALYSIS
17.20.3 GEOGRAPHIC PRESENCE
17.20.4 PRODUCT PORTFOLIO
17.20.5 RECENT DEVELOPEMENTS
17.21 GRIFOLS, S.A.
17.21.1 COMPANY OVERVIEW
17.21.2 REVENUE ANALYSIS
17.21.3 GEOGRAPHIC PRESENCE
17.21.4 PRODUCT PORTFOLIO
17.21.5 RECENT DEVELOPEMENTS
17.22 LEICA BIOSYSTEMS NUSSLOCH GMBH (DANAHER CORPORATION)
17.22.1 COMPANY OVERVIEW
17.22.2 REVENUE ANALYSIS
17.22.3 GEOGRAPHIC PRESENCE
17.22.4 PRODUCT PORTFOLIO
17.22.5 RECENT DEVELOPEMENTS
17.23 ANALYTIK JENA GMBH
17.23.1 COMPANY OVERVIEW
17.23.2 REVENUE ANALYSIS
17.23.3 GEOGRAPHIC PRESENCE
17.23.4 PRODUCT PORTFOLIO
17.23.5 RECENT DEVELOPEMENTS
17.24 BIOCARTIS
17.24.1 COMPANY OVERVIEW
17.24.2 REVENUE ANALYSIS
17.24.3 GEOGRAPHIC PRESENCE
17.24.4 PRODUCT PORTFOLIO
17.24.5 RECENT DEVELOPEMENTS
17.25 LUMINEX CORPORATION
17.25.1 COMPANY OVERVIEW
17.25.2 REVENUE ANALYSIS
17.25.3 GEOGRAPHIC PRESENCE
17.25.4 PRODUCT PORTFOLIO
17.25.5 RECENT DEVELOPEMENTS
18 RELATED REPORTS
19 CONCLUSION
20 QUESTIONNAIRE
21 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












