Global Oncology Small Molecule Api Market
Market Size in USD Billion
CAGR :
% 
 USD
18.01 Billion
USD
32.13 Billion
2024
2032
USD
18.01 Billion
USD
32.13 Billion
2024
2032
| 2025 –2032 | |
| USD 18.01 Billion | |
| USD 32.13 Billion | |
|
|
|
|
Oncology Small Molecule API Market Analysis
The oncology small molecule API market is experiencing significant growth driven by the increasing prevalence of cancer worldwide and the rising demand for targeted therapies. Small molecules have emerged as an effective approach to treating various types of cancer due to their ability to precisely target cancer cells while minimizing damage to surrounding healthy tissue. This market's expansion is also fueled by continuous advancements in drug development, with a focus on precision medicine and the increasing adoption of novel therapies such as kinase inhibitors and immune modulators.
Key factors contributing to market growth include the growing investments in research and development by pharmaceutical companies, the continuous pipeline of oncology drugs, and the shift toward personalized treatment regimens. In addition, the rise of biologics and biosimilars has spurred innovation in the small molecule API segment, as pharmaceutical companies seek to offer alternative treatments with fewer side effects and enhanced efficacy.
The market is also benefiting from the increasing approval of new oncology small molecule drugs by regulatory authorities, improving patient access to cutting-edge treatments. Moreover, the rising preference for combination therapies that integrate small molecules with other therapeutic modalities, such as immunotherapy, further strengthens the market's prospects.
Oncology Small Molecule API Market Size
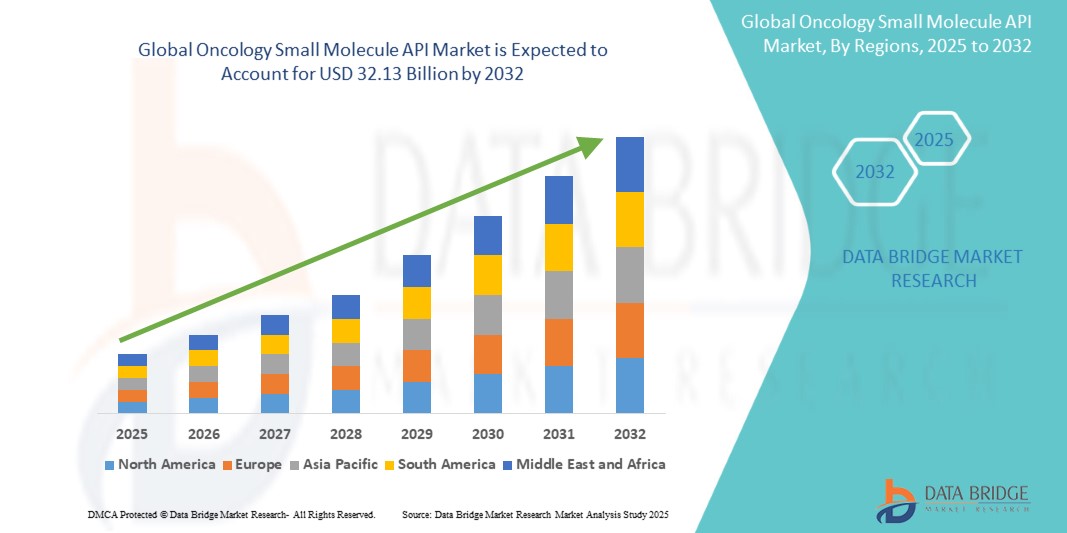
The global oncology small molecule API market size was valued at USD 18.01 billion in 2024 and is projected to reach USD 32.13 billion by 2032, with a CAGR of 7.50% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Oncology Small Molecule API Market Trends
“Growing Demand for Effective Cancer Treatments”
The oncology small molecule API market is flourishing primarily due to the growing demand for effective cancer treatments and advancements in drug development. Small molecules, which target specific molecular pathways involved in cancer progression, have proven highly effective in treating various cancer types. Their ability to precisely target cancer cells while minimizing damage to healthy tissue has made them increasingly popular in precision medicine approaches. Furthermore, continuous innovation in oncology therapies, such as kinase inhibitors and immune-modulating small molecules, has expanded the range of treatment options. The rising focus on personalized cancer therapies, which tailor treatments to individual genetic profiles, also drives market growth. In addition, the increasing approval of new oncology drugs and the strategic investments by pharmaceutical companies in R&D further boost the market. Together, these factors are propelling the demand for small molecule APIs, making them a crucial component of the evolving oncology treatment landscape.
Report Scope and Oncology Small Molecule API Market Segmentation
|
Attributes |
Oncology Small Molecule API Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Indonesia, Rest of Asia-Pacific, Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
Abbott (U.S.), Accord Healthcare (UK), AbbVie Inc. (U.S.), Apotex Inc. (Canada), AstraZeneca (UK), Bayer AG (Germany), Boehringer Ingelheim International GmbH (Germany), Bristol-Myers Squibb Company (U.S.), DAIICHI SANKYO COMPANY, LIMITED (Japan), Dr. Reddy’s Laboratories Ltd. (India), F. Hoffmann-La Roche Ltd (Switzerland), Fresenius Kabi AG (Germany), Genentech, Inc. (U.S.), Gilead Sciences, Inc. (U.S.), GSK plc. (UK), Johnson & Johnson Services, Inc. (U.S.), Lilly (U.S.), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Sun Pharmaceutical Industries Ltd. (India), and Viatris Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Oncology Small Molecule API Market Definition
Oncology small molecule active pharmaceutical ingredients (API) refer to the active ingredients in pharmaceutical drugs used to treat cancer, which are small, chemically synthesized molecules. These molecules work by targeting specific biological processes or pathways that promote cancer cell growth and survival. Unlike biologic therapies, which are made from living organisms, small molecules are typically manufactured through chemical processes and can be administered orally or intravenously.
Oncology Small Molecule API Market Dynamics
Drivers
- Increasing Prevalence of Cancer
The rising global incidence of cancer significantly drives the demand for oncology small molecule APIs. As cancers such as lung, breast, and colorectal become more common, the need for effective treatments intensifies. Small molecule APIs such as cisplatin, used in chemotherapy, and targeted therapies such as imatinib for chronic myelogenous leukemia, are essential in managing these conditions. For instance, the global rise in breast cancer cases has led to an increased use of small molecule drugs like letrozole. This surge in usage has sparked ongoing research into optimizing treatment efficacy and minimizing side effects for better patient outcomes. The growing patient population is leading to higher drug consumption, thereby boosting the market for small molecule APIs. With continued cancer growth, this trend is expected to sustain the demand for these drugs.
- Advancements in Drug Development
Ongoing innovations in drug development are propelling the oncology small molecule API market. Advances such as the creation of kinase inhibitors such as gefitinib for non-small cell lung cancer (NSCLC) and immune-modulating small molecules are expanding treatment options. For instance, the approval of osimertinib, a targeted therapy for EGFR-mutated NSCLC, has significantly improved patient outcomes. These targeted therapies focus on specific molecular pathways to inhibit cancer cell growth, offering more effective and personalized treatments. Furthermore, the approval of venetoclax for chronic lymphocytic leukemia (CLL) continues to fuel demand. As drug development accelerates, the market for oncology small molecule APIs is expected to grow substantially.
Opportunities
- Personalized Medicine and Targeted Therapies
The growing focus on personalized medicine presents a significant opportunity in the oncology small molecule API market. Tailoring cancer treatments based on an individual’s genetic profile allows for more effective therapies with fewer side effects. For instance, targeted therapies such as imatinib for chronic myelogenous leukemia (CML) or osimertinib for EGFR-mutated non-small cell lung cancer (NSCLC) are improving treatment outcomes. These targeted therapies offer more precise, personalized treatment options, leading to improved survival rates and reduced adverse effects for patients. As genetic testing becomes more widespread, the demand for small molecules that target specific genetic mutations is expected to rise, offering substantial growth potential in this market.
- Expansion of Combination Therapies
Another key opportunity in the oncology small molecule API market lies in the growing adoption of combination therapies, which are revolutionizing cancer treatment. By combining small molecules with immunotherapies or other therapeutic modalities, these combinations enhance treatment efficacy, improve patient outcomes, and tackle cancer’s complex and evolving nature. For instance, the combination of small molecule inhibitors like venetoclax with immunotherapies is showing significant promise in treating hematologic cancers such as chronic lymphocytic leukemia (CLL). As new combination therapies are developed and gain approval, the demand for small molecule APIs will continue to surge, fostering market growth and offering potential for innovative treatments.
Restraints/Challenges
- High Development and Manufacturing Costs
One major restraint in the oncology small molecule API market is the high cost of drug development and manufacturing. The complex and lengthy process of creating small molecule oncology drugs, from research to clinical trials, requires significant investment. For instance, the development of targeted therapies such as osimertinib or venetoclax involves extensive research and clinical validation, making these drugs expensive to bring to market. These high costs can limit accessibility and slow the development of new therapies, presenting a challenge for both manufacturers and patients.
- Stringent Regulatory Hurdles
Stringent regulatory requirements are another challenge faced by the oncology small molecule API market. Navigating the complex approval process for new drugs, particularly in oncology, can delay market access and increase development costs. For instance, while imatinib and other targeted therapies have received approval, their development was subjected to rigorous regulatory scrutiny. These hurdles can limit the speed at which new treatments become available, creating challenges for companies trying to meet patient demand quickly.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Oncology Small Molecule API Market Scope
The market is segmented on the basis of type of cancer, API classification type, API type, manufacturer type, by end use and type of synthesis. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type of Cancer
- Breast
- Cervical
- Lung
- Liver
- Colorectal
- Others
API Classification Type
- Thalidomide Analogues
- Alkalyting agents
- Androgen receptors
- Folate Analog Metabolic
- Nucleoside Metabolic
- Microtubule
- Proteasome Inhibitors
API Type
- Innovative Oncology APIs
- Generic Oncology APIs
Manufacturer Type
- Captive
- Merchant
End Use
- Hospitals
- Diagnostic laboratories
- Diagnostic imaging centers
- Academia
- Specialty clinics
- Other end users
Type of Synthesis
- Synthetic Oncology APIs
- Biotech Oncology APIs
Oncology Small Molecule API Market Regional Analysis
The market is analysed and market size insights and trends are provided by type of cancer, API classification type, API type, manufacturer type, end use, and type of synthesis as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Indonesia, rest of Asia-Pacific, Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, rest of Middle East and Africa, Brazil, Argentina and rest of South America.
The North American region is expected to dominate the oncology small molecule API market. This is driven by the high prevalence of cancer and a strong healthcare infrastructure that supports the rapid adoption of innovative therapies, particularly the U.S.The presence of major pharmaceutical companies such as Pfizer and Merck, which lead oncology drug development, also boosts market growth. In addition, favorable regulatory environments, such as initiatives from the U.S. FDA to expedite cancer drug approvals, further accelerate the availability of new treatments. These factors position North America as the dominant region in the oncology small molecule API market.
The Asia-Pacific (APAC) region is expected to exhibit the highest growth rate in the oncology small molecule API market. The increasing prevalence of cancer in countries such as China and India, along with improving healthcare infrastructure, is driving the demand for effective cancer treatments. In addition, the rising adoption of advanced therapies, greater access to healthcare, and government initiatives to enhance medical research contribute to market growth. The growing pharmaceutical industry in the region, coupled with cost-effective production capabilities, also supports the expansion of small molecule APIs, making APAC a rapidly growing market for oncology treatments.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Oncology Small Molecule API Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Oncology Small Molecule API Market Leaders Operating in the Market Are:
- Abbott (U.S.)
- Accord Healthcare (UK)
- AbbVie Inc. (U.S.)
- Apotex Inc. (Canada)
- AstraZeneca (UK)
- Bayer AG (Germany)
- Boehringer Ingelheim International GmbH (Germany)
- Bristol-Myers Squibb Company (U.S.)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- Dr. Reddy’s Laboratories Ltd. (India)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Fresenius Kabi AG (Germany)
- Genentech, Inc. (U.S.)
- Gilead Sciences, Inc. (U.S.)
- GSK plc. (UK)
- Johnson & Johnson Services, Inc. (U.S.)
- Lilly (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Viatris Inc. (U.S.)
Latest Developments in Oncology Small Molecule API Market
- In January 2025, GSK plc announced that the US Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation to GSK5764227 (GSK’227), its B7-H3-targeted antibody-drug conjugate (ADC). The drug is being evaluated for the treatment of adult patients with relapsed or refractory osteosarcoma (bone cancer) who have not responded to at least two prior lines of therapy. Osteosarcoma mainly affects children and young adults and is the most common primary bone cancer
- In January 2025, Pfizer Inc. announced favorable topline results from its pivotal Phase 3 CREST trial, which assessed sasanlimab, an investigational anti-PD-1 monoclonal antibody (mAb), in combination with Bacillus Calmette-Guérin (BCG) as induction therapy, with or without maintenance, for patients with BCG-naïve, high-risk non-muscle invasive bladder cancer (NMIBC)
- In December 2024, GSK plc announced that the US FDA has granted Breakthrough Therapy Designation to Jemperli (dostarlimab-gxly) for the treatment of patients with locally advanced mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) rectal cancer. This designation is intended to accelerate the development and review of drugs that show promise in treating serious conditions, particularly when preliminary clinical evidence suggests a significant improvement over existing therapies
- In October 2024, Sun Pharmaceutical Industries has announced a global licensing agreement with the Italian-Swiss company Philogen for an anti-cancer drug. Under the terms of the deal, Sun Pharma will hold exclusive worldwide rights to commercialize Fibromun, which is currently undergoing registration trials by Philogen for the treatment of soft tissue sarcoma and glioblastoma
- In October 2024, AbbVie and EvolveImmune Therapeutics announced a collaboration and option-to-license agreement aimed at developing multispecific biologics targeting multiple oncology indications. The discovery partnership will utilize EvolveImmune’s T-cell engager platform to create innovative antibody-based therapies for both solid and hematologic cancers. EvolveImmune's proprietary EVOLVE platform is designed to provide potent, selective, and integrated T-cell co-stimulation, enhancing and sustaining the tumor-killing potential of T-cells
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.