Global Ophthalmology Small Molecule Api Market
Market Size in USD Billion
CAGR :
% 
 USD
4.88 Billion
USD
7.49 Billion
2024
2032
USD
4.88 Billion
USD
7.49 Billion
2024
2032
| 2025 –2032 | |
| USD 4.88 Billion | |
| USD 7.49 Billion | |
|
|
|
Ophthalmology Small Molecule API Market Analysis
The ophthalmology small molecule API market is witnessing robust growth, driven by the increasing prevalence of ocular diseases, particularly age-related macular degeneration (AMD), diabetic retinopathy, glaucoma, and dry eye disease. As the global population ages, the demand for effective treatments for these chronic conditions is rising, propelling the market forward. Small molecule drugs offer advantages such as ease of administration, lower cost compared to biologics, and broader accessibility, making them highly attractive for both patients and healthcare systems.
The approval of novel small molecule drugs targeting various pathways in the eye, such as tyrosine kinase inhibitors for diabetic retinopathy and Rho kinase inhibitors for glaucoma, is expanding treatment options and improving patient outcomes. Furthermore, the growing trend of personalized medicine in ophthalmology is driving research into small molecules tailored to specific genetic and disease profiles.
Ophthalmology Small Molecule API Market Size
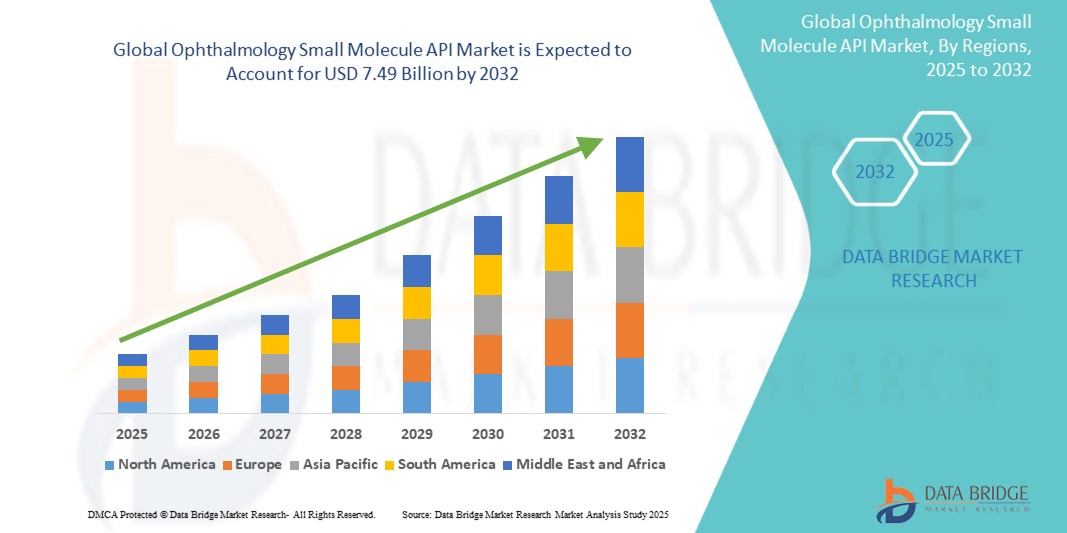
The global ophthalmology small molecule API market size was valued at USD 4.88 billion in 2024 and is projected to reach USD 7.49 billion by 2032, with a CAGR of 5.50% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Ophthalmology Small Molecule API Market Trends
“Increasing Prevalence of Ocular Diseases”
The main reason for the growth of the ophthalmology small molecule API market is the increasing prevalence of ocular diseases, particularly age-related macular degeneration (AMD), diabetic retinopathy, glaucoma, and dry eye disease. As the global population ages and with the rise in chronic conditions such as diabetes, the demand for effective and affordable treatments for these diseases is growing rapidly.
Small molecule drugs offer significant advantages, including lower cost compared to biologics, ease of administration, and wider accessibility, making them an attractive treatment option for both patients and healthcare systems, especially in regions with budget constraints. In addition, the development of novel small molecule therapies targeting specific pathways in the eye, such as Rho kinase inhibitors for glaucoma and tyrosine kinase inhibitors for diabetic retinopathy, is expanding the therapeutic options available, improving patient outcomes, and fueling market growth. These factors, combined with increasing healthcare access and awareness, particularly in emerging markets, are driving the demand and growth of the ophthalmology small molecule API ma
Report Scope and Ophthalmology Small Molecule API Market Segmentation
|
Attributes |
Ophthalmology Small Molecule API Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Abbott (U.S.), AbbVie Inc. (U.S.), Amgen Inc. (U.S.), Apotex Inc. (Canada), AstraZeneca (United Kingdom), Bayer AG (Germany), Bausch + Lomb (Canada), BioCryst Pharmaceuticals, Inc. (U.S.), Boehringer Ingelheim International GmbH (Germany), Bristol-Myers Squibb Company (U.S.), Cipla (India), DAIICHI SANKYO COMPANY, LIMITED (Japan), F. Hoffmann-La Roche Ltd (Switzerland), Fresenius Kabi AG (Germany), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Regeneron Pharmaceuticals Inc. (U.S.), Ripple Therapeutics Corporation (Canada), Santen Pharmaceutical Co., Ltd. (Japan), and Viatris Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Ophthalmology Small Molecule API Market Definition
Ophthalmology small molecule API refers to active pharmaceutical ingredients used in small molecule drugs designed for the treatment of eye diseases. These low molecular weight compounds target specific biological processes to address conditions of eyes.
Ophthalmology Small Molecule API Market Dynamics
Drivers
- Cost-Effectiveness of Small Molecules
Small molecules are generally more affordable than biologic therapies, making them an appealing choice for both patients and healthcare systems, particularly in emerging markets. Their lower cost facilitates broader accessibility and increased adoption, thereby supporting the growth of the ophthalmology small molecule API market. For instance, Methazolamide, a small molecule used in the management of glaucoma, is a cost-effective alternative to biologics such as anti-VEGF inhibitors (e.g., ranibizumab for age-related macular degeneration), particularly in low-income regions where access to high-cost biologics may be limited. This cost-effectiveness, coupled with the growing need for accessible treatments in emerging markets, positions small molecule ophthalmic drugs as a key driver of market expansion, offering a sustainable solution for the treatment of a range of eye conditions.
- Technological Advancements in Drug Delivery
Innovations in drug delivery systems, such as sustained-release formulations, are enhancing the efficacy and bioavailability of small molecule ophthalmic drugs. These advancements not only improve the therapeutic outcomes but also boost patient compliance, particularly for chronic conditions requiring long-term treatment. For instance, Travoprost, a prostaglandin analogue used in the management of glaucoma, has been developed into sustained-release formulations, ensuring consistent therapeutic effects and promoting better adherence to treatment regimens. These advancements in drug delivery systems are driving the market by making small molecule ophthalmic drugs more effective and patient-friendly, thus supporting long-term management of eye diseases and broadening their adoption in the global ophthalmic therapeutics market.
Opportunities
- Development of New Therapies for Unmet Needs
There is an increasing focus on developing small molecule drugs to address rare or underserved ophthalmic conditions. Diseases such as dry eye disease, uveitis, and retinitis pigmentosa remain challenging to treat, creating opportunities for small molecule APIs to fill these therapeutic gaps. For instance, Lifitegrast (Xiidra), a small molecule developed for dry eye disease, targets inflammation triggered by T-cell activation, offering new treatment options for patients suffering from DED. This growing focus on underserved ophthalmic conditions presents significant opportunities for the small molecule API market, driving innovation and expanding treatment options for a broader patient population, ultimately fueling market growth.
- Increasing Market Penetration in Emerging Markets
As healthcare infrastructure continues to develop in regions such as Asia-Pacific, Latin America, and parts of Africa, small molecule ophthalmic drugs have a substantial opportunity to capture market share. These regions represent emerging markets where affordable treatments are in high demand to address increasing eye care needs. For instance, small molecules such as timolol and latanoprost are commonly used in countries such as India and Brazil, where limited healthcare budgets make biologic treatments less accessible for a large portion of the population. This growing demand for affordable, effective ophthalmic treatments in these regions presents a significant opportunity for small molecule drugs to expand their market presence, ultimately improving access to eye care and driving market growth.
Restraints/Challenges
- Competition from biologic therapies
Competition from biologic therapies, particularly monoclonal antibodies such as ranibizumab (for AMD) and aflibercept, poses a challenge for small molecule ophthalmic drugs. These biologics often offer superior efficacy in certain ophthalmic conditions, especially those resistant to small molecule treatments. The increasing preference for biologics, particularly in the management of retinal diseases, can restrict the market share of small molecule ophthalmic APIs. For instance, Anti-VEGF drugs such as ranibizumab and bevacizumab have become the gold standard for treating wet AMD, limiting the growth opportunities for small molecules in this area. As a result, the dominance of biologics in treating complex ophthalmic conditions poses a significant restraint on the market growth of small molecule ophthalmic APIs, especially in indications such as wet AMD, where biologics have established themselves as the preferred treatment.
- Barriers in Drug Delivery to the Eye
A key challenge for small molecule ophthalmic APIs is the difficulty in achieving effective drug delivery to the target tissues, particularly the retina and posterior segment of the eye. Conventional delivery methods, such as eye drops, often struggle to reach deeper eye structures, limiting their efficacy for conditions affecting these areas. For instance, topical corticosteroids such as prednisolone may fail to adequately penetrate and treat deeper ocular tissues involved in conditions like macular edema. This challenge of effective drug delivery underscores a significant limitation for the growth and adoption of small molecule ophthalmic APIs, potentially hindering their ability to compete with more targeted treatments like biologics.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Ophthalmology Small Molecule API Market Scope
The market is segmented on the basis of drug class, dosage form, disease indication, route of administration and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class:
- Anti-inflammatory
- Anti-infectives
- Anti-VEGF
- Anti-glaucoma
- Beta Blockers,
- Prostaglandins Analogs
- Alpha Adrenergic Agonists
- Others
- Others
Dosage Form
- Gels
- Eye Solutions & Suspensions
- Capsules
- Tablets
- Eye Drops
- Ointments
Disease Indication
- Glaucoma
- Open Angle Glaucoma
- Angle Closure Glaucoma
- Dry Eye Disease
- Retinal Diseases
- Diabetic Macular Edema (DME)
- Macular Degeneration (AMD)
- Diabetic Retinopathy (DR)
- Retinal Vein Occlusion (RVO)
- Others
- Allergy
- Infections
Route of Administration
- Topical
- Local Ocular
- Systemic
Distribution Channel
- Hospital Pharmacies
- Retail & Online Pharmacies
Ophthalmology Small Molecule API Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, drug class, dosage form, disease indication, route of administration, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America held the largest market share and revenue in the previous year. The region is expected to maintain its dominance in the coming years, driven by the increasing prevalence of ophthalmic disorders, along with higher rates of diagnosis and treatment. In addition, the rising healthcare expenditure dedicated to eye care and health, as well as the availability of adequate reimbursement for various ophthalmic conditions, are fostering the adoption of advanced and novel treatments in key countries across the region.
Asia Pacific is expected to grow with highest CAGR during the forecast period, driven by the increasing prevalence of age-related ophthalmic conditions, particularly among the geriatric population. In addition, strategic initiatives by companies operating in the region to enhance the availability of various ophthalmic drugs, along with rising patient awareness about new treatments, are fueling market expansion. For instance, in May 2022, Visus Therapeutics, Inc., a company focused on ophthalmic therapies, partnered with Zhaoke Ophthalmology Limited to commercialize BRIMOCHOL PF and Carbachol PF in Greater China, South Korea, and select Southeast Asian markets.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Ophthalmology Small Molecule API Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Ophthalmology Small Molecule API Market Leaders Operating in the Market Are:
- Abbott (U.S.)
- AbbVie Inc. (U.S.)
- Amgen Inc. (U.S.)
- Apotex Inc. (Canada)
- AstraZeneca (U.K.)
- Bayer AG (Germany)
- Bausch + Lomb (Canada)
- BioCryst Pharmaceuticals, Inc. (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- Bristol-Myers Squibb Company (U.S.)
- Cipla (India)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Fresenius Kabi AG (Germany)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Regeneron Pharmaceuticals Inc. (U.S.)
- Ripple Therapeutics Corporation (Canada)
- Santen Pharmaceutical Co., Ltd. (Japan)
- Viatris Inc. (U.S.)
Latest Developments in Ophthalmology Small Molecule API Market
- In December 2024, Santen Pharmaceutical Co., Ltd. announced that it has received approval for the manufacturing and marketing of RYJUSEA, Mini ophthalmic solution 0.025% (generic name: atropine sulfate hydrate) in Japan, aimed at slowing the progression of myopia. This product is the first ophthalmic solution approved in Japan for suppressing myopia progression
- In April 2024, Viatris Inc. has announced the U.S. commercial launch of RYZUMVI (phentolamine ophthalmic solution) 0.75%, a treatment for pharmacologically-induced mydriasis caused by adrenergic agonists (such as phenylephrine) or parasympatholytic agents (such as tropicamide). RYZUMVI is now the only FDA-approved eye drop in the United States specifically designed to reverse pupil dilation
- In March 2024, Roche Pharma India announced the launch of Vabysmo (faricimab) for the treatment of neovascular or “wet” age-related macular degeneration (nAMD) and diabetic macular edema (DME). Vabysmo is the only dual-pathway inhibitor that specifically targets and blocks two key disease pathways associated with various vision-threatening retinal conditions
- In June 2023, Bausch + Lomb Corporation announced that it has reached a definitive agreement with Novartis to acquire XIIDRA (lifitegrast ophthalmic solution) 5%, a non-steroidal eye drop specifically approved for treating the signs and symptoms of dry eye disease (DED), with a focus on addressing the inflammation associated with the condition
- In June 2022, Novartis announced its acquisition of Kedalion Therapeutics and its innovative AcuStream™ technology, a device designed to enable precise dosing and accurate delivery of certain topical ophthalmic medications to the eye. This acquisition strengthens Novartis' ophthalmic portfolio and supports its ongoing efforts to explore transformative ophthalmic solutions aimed at addressing unmet patient needs in front-of-eye conditions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.