Global Oral Antidiabetic Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
45.00 Billion
USD
57.00 Billion
2021
2029
USD
45.00 Billion
USD
57.00 Billion
2021
2029
| 2022 –2029 | |
| USD 45.00 Billion | |
| USD 57.00 Billion | |
|
|
|
|
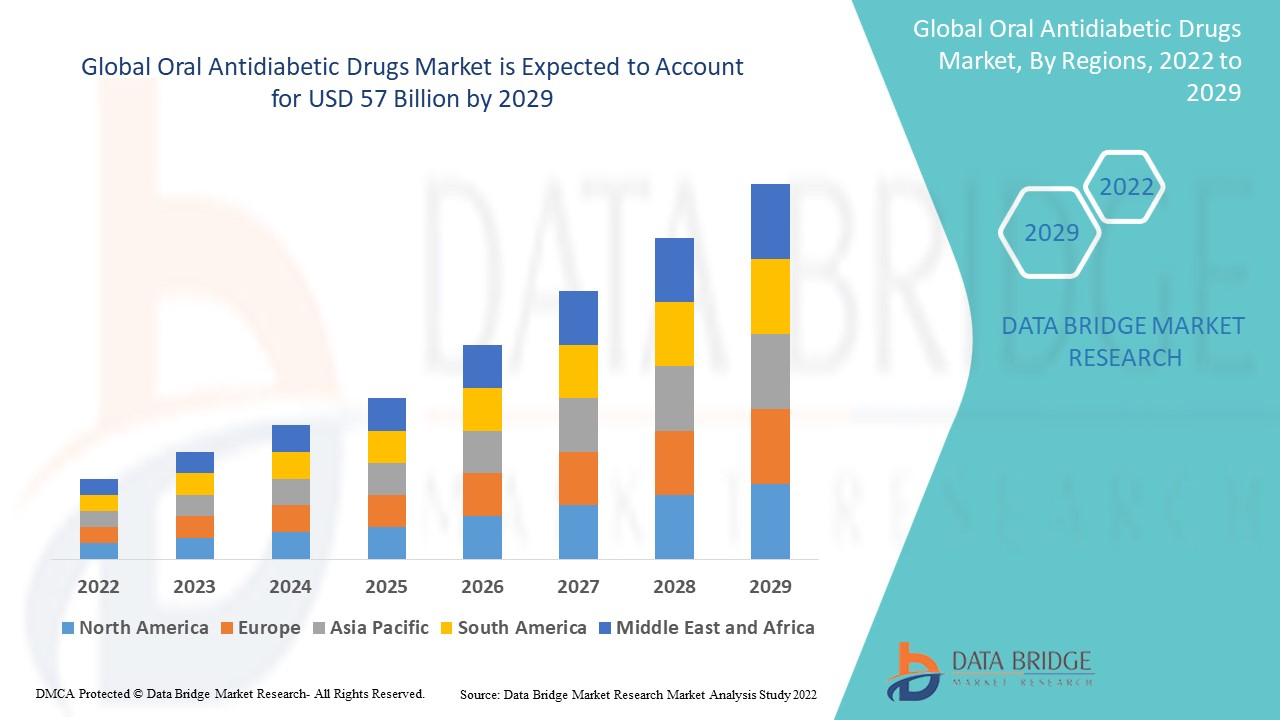
Oral Antidiabetic Drugs Market Analysis and Size
The global oral antidiabetic drugs market is expected to witness significant growth during the forecast period. Increasing cases of type 2 diabetes boosts the oral antidiabetic drugs market. The adaptation of unhealthy lifestyle, environment pollution and family history with diabetes disease also boost up the oral antidiabetic drugs market growth. Many major market players are contributing a lot in drug discovery and development. COVID-19 also had a major impact on the market growth.
Data Bridge Market Research analyses a growth rate in the global oral antidiabetic drugs market in the forecast period 2022-2029. The expected CAGR of global oral antidiabetic drugs market is tend to be around 3% in the mentioned forecast period. The market was valued at USD 45 billion in 2021, and it would grow upto USD 57 billion by 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Market Definition
Diabetes is a type of disease with high glucose level in the blood resulting deficiency of insulin in the body. Drugs which are used for the treatment of diabetes called as antidiabetic drugs. It reduces blood glucose level by activation of glycogen phosphorylase, and gluconeogenic enzymes, revealed decreased rates of glycogenolysis and gluconeogenesis. It is of great importance to the healthcare sector and thus is expected to rise high in the forecast period.
Report Scope and Market Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Drug Class (Biguanides, Thiazolidinediones, Dipeptidyl Peptidase IV Inhibitors, α-Glucosidase Inhibitors, Insulin Secretagogues, Amylin Analog, Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors, Glucagon-Like Peptide-1 Receptor Agonists, Others), End-Users (Hospitals, Homecare, Speciality Centres, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Pfizer Inc (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Mylan N.V. (U.S.), Fresenius Kabi AG (Germany), Hikma Pharmaceuticals PLC (U.K.), Novartis AG (Switzerland), Teva Pharmaceutical Industries Ltd. (Israel), Bristol Myers Squibb Company (U.S.) GSK Plc. (U.S.), Bayer AG (Germany) |
|
Market Opportunities |
|
Global Oral Antidiabetic Drugs Market Dynamics
Drivers
- Rising Incidence of Diabetes
The adult diabetes population in 2021 was approximately 537 million, and this number is going to rise high to 643 million in 2030. There are several factors such as obesity, unhealthy diet, and physical inactivity because of which the rate of newly diagnosed cases of Type 1 and Type 2 diabetes is seen to increase. Thus, this boosts the market growth.
Opportunities
- Increasing Demand for Retail Pharmacies
Rise in the number of open angle glaucoma therapeutics being delivered through these retail pharmacies and increase in the number of retail pharmacies in highly develped countries create opportunities for the market growth. Additionally, patients prefer retail pharmacies for purchasing drugs, as these are easily accessible.
- Increasing Demand for Metformin
Among all the other drugs, metformin is still the most prescribed oral antidiabetic medication globally with a prescription rate of 45–50% of all prescriptions and also taken by over 150 million people each year. There are some long-term positive experiences with the use of metformin which are strong evidence of clinical efficacy, safety, low cost, general availability, high adherence rate, and cost-effectiveness. Thus, it creates opportunity for the market growth.
Restraints/Challenges
- Side Effects of Oral Antidiabetes drugs
There are various side effects that are associated with the use of oral antidiabetes drugs such as nausea, gas, bloating, diarrhea, b12 deficiency, and an upset stomach. This creates hindrance for the market.
- High Cost
The huge expenditure of the treatment methods surely hamper the market growth.
This global oral antidiabetic drugs market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global oral antidiabetic drugs market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Oral Antidiabetic Drugs Market
The incidence of diabetes in people hospitalized with COVID-19 infection and the physicians' understanding that improved glycemic control might improve outcomes and reduce the hospital stay in patients with COVID-19 have truly underlined the importance of the oral anti-diabetic drugs market. People with diabetes tend to have a weaker immune system, in this regard, the COVID-19 complication exaggerates the condition, and the immune system gets weaker. Thus, COVID-19 surely increased the oral antidiabetic drugs market.
Global Oral Antidiabetic Drugs Market Scope
The global oral antidiabetic drugs market is segmented on the basis of drug class, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class
- Biguanides
- Thiazolidinediones
- Dipeptidyl Peptidase IV Inhibitors, α-Glucosidase Inhibitors
- Insulin Secretagogues
- Amylin Analog,
- Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors
- Glucagon-Like Peptide-1 Receptor Agonists
- Others
End-Users
- Hospitals
- Homecare
- Speciality Centres
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Oral Antidiabetic Drugs Market Regional Analysis/Insights
The global oral antidiabetic drugs market is analysed and market size insights and trends are provided by drug class, distribution channel and end-user as referenced above.
The major countries covered in the global oral antidiabetic drugs market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to have the highest market growth due to rapidly increasing smoking & obesity population and presence of key manufacture of the product.
Asia-Pacific dominates the market due to enhanced prevalence of diabetes and related disorders and number of generic drugs.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Oral Antidiabetic Drugs Market Share Analysis
The global oral antidiabetic drugs market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global oral antidiabetic drugs market.
Key players operating in the global oral antidiabetic drugs market include:
- Pfizer Inc (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Mylan N.V. (U.S.)
- Fresenius Kabi AG (Germany)
- Novartis AG (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Bristol Myers Squibb Company (U.S.)
- GSK Plc. (U.S.)
- Bayer AG (Germany)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ORAL ANTIDIABETIC DRUGS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL XX SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
6.6 TREATMENT PARADIGM AND OUTLOOK
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR XX
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
10.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.13.1 FORECAST MARKET OUTLOOK
10.13.2 CROSS COMPETITION
10.13.3 THERAPEUTIC PORTFOLIO
10.13.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 MARKET OVERVIEW
13.1 DRIVERS
13.2 RESTRAINTS
13.3 OPPORTUNITIES
13.4 CHALLENGES
14 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, BY DRUG CLASS
14.1 OVERVIEW
14.2 BIGUANIDES (METFORMIN)
14.2.1 BIGUANIDES (METFORMIN), BY TYPE
14.2.1.1. MONOTHERAPY
14.2.1.2. COMBINATION THERAPY
14.2.2 BIGUANIDES, BY DRUG TYPE
14.2.2.1. GENERIC
14.2.2.2. BRANDED
14.2.2.2.1. FORTAMET
14.2.2.2.2. GLUCOPHAGE
14.2.2.2.3. GLUCOPHAGE XR
14.2.2.2.4. GLUMETZA
14.2.2.2.5. RIOMET
14.2.2.2.6. OTHERS (IF ANY)
14.3 THIAZOLIDINEDIONES
14.3.1 THIAZOLIDINEDIONES, BY TYPE
14.3.1.1. ROSIGLITAZONE
14.3.1.2. PIOGLITAZONE
14.3.2 THIAZOLIDINEDIONES, BY DRUG TYPE
14.3.2.1. GENERIC
14.3.2.2. BRANDED
14.3.2.2.1. ACTOS
14.3.2.2.2. AVANDIA
14.3.2.2.3. OTHERS (IF ANY)
14.4 DIPEPTIDYL PEPTIDASE IV INHIBITORS
14.4.1 DIPEPTIDYL PEPTIDASE IV INHIBITORS, BY TYPE
14.4.1.1. SITAGLIPTIN
14.4.1.2. LINAGLIPTIN
14.4.1.3. SAXAGLIPTIN
14.4.1.4. ALOGLIPTIN
14.4.2 DIPEPTIDYL PEPTIDASE IV INHIBITORS, BY DRUG TYPE
14.4.2.1. GENERIC
14.4.2.2. BRANDED
14.4.2.2.1. JANUVIA
14.4.2.2.2. TRADJENTA
14.4.2.2.3. ONGLYZA
14.4.2.2.4. NESINA
14.4.2.2.5. OTHERS (IF ANY)
14.5 Α-GLUCOSIDASE INHIBITORS
14.5.1 A-GLUCOSIDASE INHIBITORS, BY TYPE
14.5.1.1. ACARBOSE
14.5.1.2. MIGLITOL
14.5.2 A-GLUCOSIDASE INHIBITORS, BY DRUG TYPE
14.5.2.1. GENERIC
14.5.2.2. BRANDED
14.5.2.2.1. PRECOSE
14.5.2.2.2. GLYSET
14.5.2.2.3. OTHERS (IF ANY)
14.6 INSULIN SECRETAGOGUES
14.6.1 SULFONYLUREAS
14.6.1.1. SULFONYLUREAS, BY TYPE
14.6.1.1.1. FIRST GENERATION
14.6.1.1.1.1 ACETOHEXAMIDE
14.6.1.1.1.2 CHLORPROPAMIDE
14.6.1.1.1.3 TOLAZAMIDE
14.6.1.1.1.4 TOLBUTAMIDE
14.6.1.1.2. SECOND GENERATION
14.6.1.1.2.1 GLIPIZIDE
14.6.1.1.2.2 GLYBURIDE
14.6.1.1.2.3 GLICLAZIDE
14.6.1.1.2.4 GLIMEPIRIDE
14.6.1.2. SULFONYLUREAS, BY DRUG TYPE
14.6.1.2.1. GENERIC
14.6.1.2.2. BRANDED
14.6.1.2.2.1 DIAMICRON
14.6.1.2.2.2 GLIBENESE
14.6.1.2.2.3 AMARYL
14.6.1.2.2.4 GLYNASE
14.6.1.2.2.5 OTHERS (IF ANY)
14.6.2 GLINIDES
14.6.2.1. GLINIDES, BY TYPE
14.6.2.1.1. REPAGLINIDE
14.6.2.1.2. NATEGLINIDE
14.6.2.2. GLINIDES, BY DRUG TYPE
14.6.2.2.1. GENERIC
14.6.2.2.2. BRANDED
14.6.2.2.2.1 PRANDIN
14.6.2.2.2.2 STARLIX
14.6.2.2.2.3 OTHERS (IF ANY)
14.7 SODIUM-GLUCOSE COTRANSPORTER-2 (SGLT2) INHIBITORS
14.7.1 SGLT2 INHIBITORS, BY TYPE
14.7.1.1. CANAGLIFLOZIN
14.7.1.2. DAPAGLIFLOZIN
14.7.1.3. EMPAGLIFLOZIN
14.7.1.4. OTHERS (IF ANY)
14.7.2 SGLT2 INHIBITORS, BY DRUG TYPE
14.7.2.1. GENERIC
14.7.2.2. BRANDED
14.7.2.2.1. FORXIGO
14.7.2.2.2. INVOKANA
14.7.2.2.3. BRENZAVVY
14.7.2.2.4. JARDIANCE
14.7.2.2.5. STEGLATRO
14.7.2.2.6. OTHERS (IF ANY)
14.8 GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONISTS
14.9 OTHERS
14.9.1 COMBINATION DRUGS
14.9.1.1. COMBINATION DRUGS, BY TYPE
14.9.1.1.1. ALOGLIPTIN/METFORMIN
14.9.1.1.2. CANAGLIFLOZIN/METFORMIN
14.9.1.1.3. DAPAGLIFLOZIN/METFORMIN
14.9.1.1.4. DAPAGLIFLOZIN/METFORMIN/SAXAGLIPTIN
14.9.1.1.5. EMPAGLIFLOZIN/LINAGLIPTIN/METFORMIN
14.9.1.1.6. EMPAGLIFLOZIN/METFORMIN
14.9.1.1.7. ERTUGLIFLOZIN/METFORMIN
14.9.1.1.8. GLIPIZIDE/METFORMIN
14.9.1.1.9. GLYBURIDE/METFORMIN
14.9.1.1.10. LINAGLIPTIN/METFORMIN
14.9.1.1.11. METFORMIN/PIOGLITAZONE
14.9.1.1.12. METFORMIN/ROSIGLITAZONE
14.9.1.1.13. METFORMIN/REPAGLINIDE
14.9.1.1.14. OTHERS
14.9.1.2. COMBINATION DRUGS, BY DRUG TYPE
14.9.1.2.1. GENERIC
14.9.1.2.2. BRANDED
14.9.1.2.2.1 KAZANO
14.9.1.2.2.2 INVOKAMET
14.9.1.2.2.3 XIGDUO XR
14.9.1.2.2.4 QTERNMET XR
14.9.1.2.2.5 TRIJARDY XR
14.9.1.2.2.6 SYNJARDY
14.9.1.2.2.7 SEGLUROMET
14.9.1.2.2.8 METAGLIP
14.9.1.2.2.9 GLUCOVANCE
14.9.1.2.2.10 JENTADUETO
14.9.1.2.2.11 JENTADUETO XR
14.9.1.2.2.12 ACTOPLUS MET
14.9.1.2.2.13 PRANDIMET
14.9.1.2.2.14 AVANDAMET
14.9.1.2.2.15 KOMBIGLYZE XR
14.9.1.2.2.16 OTHERS
14.9.2 OTHERS (IF ANY)
15 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, BY DRUG TYPE
15.1 OVERVIEW
15.2 BRANDED
15.2.1 FORTAMET
15.2.2 GLUCOPHAGE
15.2.3 GLUCOPHAGE XR
15.2.4 GLUMETZA
15.2.5 RIOMET
15.2.6 FORXIGO
15.2.7 INVOKANA
15.2.8 BRENZAVVY
15.2.9 JARDIANCE
15.2.10 STEGLATRO
15.2.11 PRANDIN
15.2.12 STARLIX
15.2.13 DIAMICRON
15.2.14 GLIBENESE
15.2.15 AMARYL
15.2.16 GLYNASE
15.2.17 OTHERS
15.3 GENERIC
16 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, BY ACTIVITY
16.1 OVERVIEW
16.2 EXTENDED RELEASE
16.3 IMMEDIATE RELEASE
17 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, BY DIABETES TYPE
17.1 OVERVIEW
17.2 TYPE 1 DIABETES
17.3 TYPE 2 DIABETES
17.4 GESTATIONAL DIABETES
18 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, BY REGIMENS
18.1 OVERVIEW
18.2 MONTHERAPY
18.3 COMBINATION THERAPY
19 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, BY POPULATION TYPE
19.1 OVERVIEW
19.2 PEDIATRIC
19.2.1 BY GENDER
19.2.1.1. MALE
19.2.1.2. FEMALE
19.3 ADULTS
19.3.1 BY GENDER
19.3.1.1. MALE
19.3.1.2. FEMALE
19.4 GERIATRIC
19.4.1 BY GENDER
19.4.1.1. MALE
19.4.1.2. FEMALE
20 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.2.1 BY TYPE
20.2.1.1. PRIVATE
20.2.1.1.1. BY TIER
20.2.1.1.1.1 TIER I
20.2.1.1.1.2 TIER II
20.2.1.1.1.3 TIER III
20.2.1.2. PUBLIC
20.2.1.2.1. BY TIER
20.2.1.2.1.1 TIER I
20.2.1.2.1.2 TIER II
20.2.1.2.1.3 TIER III
20.3 HOME HEALTHCARE
20.4 SPECIALTY CENTERS
20.5 DIABETES CARE CENTERS
20.6 AMBULATORY CENTERS
20.7 OTHERS
21 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 DIRECT TENDER
21.3 RETAIL SALES
21.3.1 HOSPITAL PHARMACY
21.3.2 RETAIL PHARMACY
21.3.3 ONLINE PHARMACY
21.4 OTHERS
22 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, SWOT AND DBMR ANALYSIS
23 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: GLOBAL
23.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS: EUROPE
23.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.5 MERGERS & ACQUISITIONS
23.6 NEW PRODUCT DEVELOPMENT & APPROVALS
23.7 EXPANSIONS
23.8 REGULATORY CHANGES
23.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, BY REGION
GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.1 NORTH AMERICA
24.1.1 U.S.
24.1.2 CANADA
24.1.3 MEXICO
24.2 EUROPE
24.2.1 GERMANY
24.2.2 U.K.
24.2.3 ITALY
24.2.4 FRANCE
24.2.5 SPAIN
24.2.6 RUSSIA
24.2.7 SWITZERLAND
24.2.8 TURKEY
24.2.9 BELGIUM
24.2.10 NETHERLANDS
24.2.11 DENMARK
24.2.12 SWEDEN
24.2.13 POLAND
24.2.14 NORWAY
24.2.15 FINLAND
24.2.16 REST OF EUROPE
24.3 ASIA-PACIFIC
24.3.1 JAPAN
24.3.2 CHINA
24.3.3 SOUTH KOREA
24.3.4 INDIA
24.3.5 SINGAPORE
24.3.6 THAILAND
24.3.7 INDONESIA
24.3.8 MALAYSIA
24.3.9 PHILIPPINES
24.3.10 AUSTRALIA
24.3.11 NEW ZEALAND
24.3.12 VIETNAM
24.3.13 TAIWAN
24.3.14 REST OF ASIA-PACIFIC
24.4 SOUTH AMERICA
24.4.1 BRAZIL
24.4.2 ARGENTINA
24.4.3 REST OF SOUTH AMERICA
24.5 MIDDLE EAST AND AFRICA
24.5.1 SOUTH AFRICA
24.5.2 EGYPT
24.5.3 BAHRAIN
24.5.4 UNITED ARAB EMIRATES
24.5.5 KUWAIT
24.5.6 OMAN
24.5.7 QATAR
24.5.8 SAUDI ARABIA
24.5.9 REST OF MEA
24.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL ORAL ANTIDIABETIC DRUGS MARKET, COMPANY PROFILE
25.1 PFIZER INC.
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 NOVARTIS
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 TEVA PHARMACEUTICALS USA, INC.
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 ASTRAZENECA
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 BAYER AG
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 SANOFI
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 ASTELLAS PHARMA INC.
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 JOHNSON & JOHNSON HEALTH CARE SYSTEMS INC.
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 NOVO NORDISK A/S
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 MERCK & CO., INC.
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 GSK PLC.
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 AJANTA PHARMAC INC.
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 LUPIN
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 AMNEAL PHARMACEUTICALS INC.
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
25.16 LES LABORATORIES SERVIER
25.16.1 COMPANY OVERVIEW
25.16.2 REVENUE ANALYSIS
25.16.3 GEOGRAPHIC PRESENCE
25.16.4 PRODUCT PORTFOLIO
25.16.5 RECENT DEVELOPMENTS
25.17 ADVACARE PHARMA
25.17.1 COMPANY OVERVIEW
25.17.2 REVENUE ANALYSIS
25.17.3 GEOGRAPHIC PRESENCE
25.17.4 PRODUCT PORTFOLIO
25.17.5 RECENT DEVELOPMENTS
25.18 WELLONA PHARMA
25.18.1 COMPANY OVERVIEW
25.18.2 REVENUE ANALYSIS
25.18.3 GEOGRAPHIC PRESENCE
25.18.4 PRODUCT PORTFOLIO
25.18.5 RECENT DEVELOPMENTS
25.19 THERACOSBIO, LLC
25.19.1 COMPANY OVERVIEW
25.19.2 REVENUE ANALYSIS
25.19.3 GEOGRAPHIC PRESENCE
25.19.4 PRODUCT PORTFOLIO
25.19.5 RECENT DEVELOPMENTS
25.2 TAKEDA PHARMACEUTICAL COMPANY LIMITED
25.20.1 COMPANY OVERVIEW
25.20.2 REVENUE ANALYSIS
25.20.3 GEOGRAPHIC PRESENCE
25.20.4 PRODUCT PORTFOLIO
25.20.5 RECENT DEVELOPMENTS
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












