Global Peanut Allergy Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
544.42 Million
USD
1,406.82 Million
2024
2032
USD
544.42 Million
USD
1,406.82 Million
2024
2032
| 2025 –2032 | |
| USD 544.42 Million | |
| USD 1,406.82 Million | |
|
|
|
|
Peanut Allergy Treatment Market Size
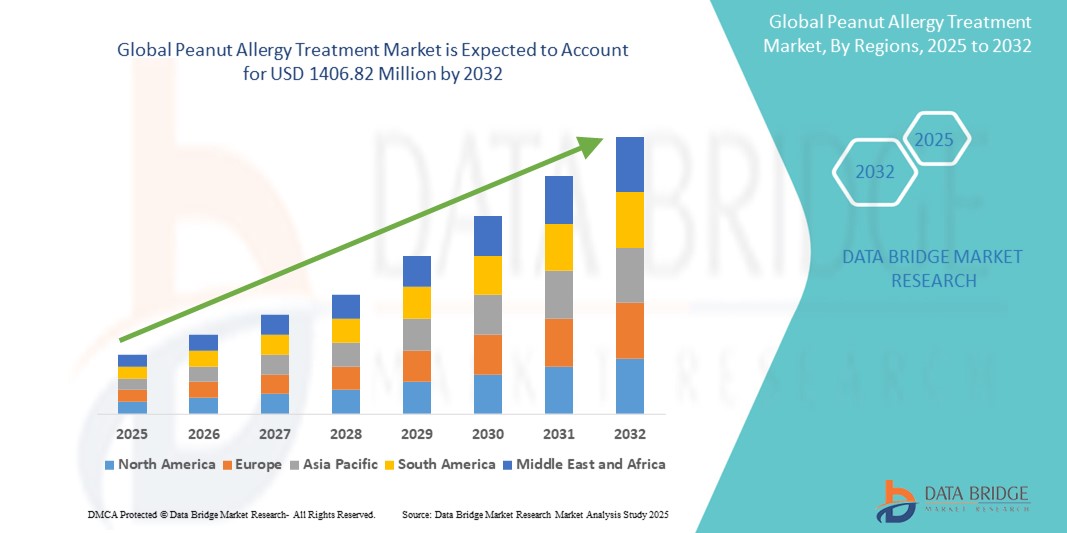
- The global peanut allergy treatment market size was valued at USD 544.42 million in 2024 and is expected to reach USD 1,406.82 million by 2032, at a CAGR of 12.6% during the forecast period
- Market growth is largely driven by the increasing global prevalence of peanut allergies, growing awareness among patients and healthcare professionals, and significant advancements in oral immunotherapy (OIT) and other investigational therapies
- The severe and life-threatening nature of allergic reactions to peanuts is establishing effective treatment and management strategies as a critical area of focus. These converging factors are accelerating the demand for and uptake of peanut allergy treatment solutions, thereby significantly boosting the industry's growth.
Peanut Allergy Treatment Market Analysis
- Peanut allergy is a severe and potentially life-threatening food allergy that affects a significant portion of the global population, particularly children. It often requires strict avoidance of peanuts and prompt emergency treatment in case of accidental exposure
- The demand for Peanut Allergy Treatment is primarily fueled by improved diagnostic capabilities leading to earlier identification, increased patient and caregiver education, and a robust pipeline of novel immunotherapies aimed at desensitization or prevention.
- North America dominates the Peanut Allergy Treatment Market with the largest revenue share of 40.01% in 2024, characterized by a high prevalence of peanut allergies, advanced healthcare infrastructure, and significant investments in food allergy research and development
- The U.S. is experiencing substantial growth in Peanut Allergy Treatment, particularly in specialized allergy clinics and academic medical centers, driven by regulatory approvals for oral immunotherapies and strong patient advocacy
- Asia-Pacific is expected to be the fastest-growing region in the Peanut Allergy Treatment Market during the forecast period due to increasing awareness, improving diagnostic facilities, and a rising incidence of food allergies in countries with large populations like China and India
- The Injectable Epinephrine segment dominates the Peanut Allergy Treatment Market with a market share of 37.32% in 2024, driven by its established efficacy in desensitizing patients to peanut allergens and its potential to significantly reduce the risk of severe reactions
Report Scope and Peanut Allergy Treatment Market Segmentation
|
Attributes |
Peanut Allergy Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Peanut Allergy Treatment Market Trends
“Emergence and Growing Adoption of Oral Immunotherapy (OIT)”
- A significant and accelerating trend in the global Peanut Allergy Treatment Market is the emergence and growing adoption of Oral Immunotherapy (OIT) as a disease-modifying treatment. OIT involves administering gradually increasing doses of peanut protein to desensitize patients, thereby reducing the severity of allergic reactions upon accidental exposure.
- This approach represents a paradigm shift from traditional avoidance strategies to proactive management.
- For instance, in April 2024, a leading allergy association reported a substantial increase in the number of allergists offering OIT for peanut allergy in clinical practice, driven by robust clinical trial data supporting its efficacy and safety. Such widespread adoption is expected to propel the Peanut Allergy Treatment industry growth in the forecast period.
- OIT offers features such as significant desensitization, improved quality of life for patients and caregivers, and reduced anxiety associated with accidental exposure, providing a compelling upgrade over strict avoidance alone. Furthermore, the growing demand for personalized medicine and the desire for proactive solutions are making OIT an integral component of comprehensive allergy management plans, offering seamless integration with patient monitoring and emergency preparedness. The convenience of at-home dosing after initial supervised administration, the potential for long-term tolerance, and the increasing availability of standardized OIT products are key factors propelling its adoption. The trend towards evidence-based guidelines and the increasing patient access to specialized allergy centers further contribute to market growth.
Peanut Allergy Treatment Market Dynamics
Driver:
“Rising Prevalence of Peanut Allergies and Increased Awareness”
- The increasing global prevalence of peanut allergies across all age groups, particularly in pediatric populations, coupled with a significant rise in awareness among parents, schools, and healthcare professionals, is a major driver for the heightened demand for Peanut Allergy Treatment.
- For instance, in April 2024, a global health report indicated a consistent upward trend in food allergy diagnoses, with peanut allergy being one of the most common and severe. Such reports by international health organizations and research institutions are expected to drive the Peanut Allergy Treatment industry growth in the forecast period.
- As patients and caregivers become more informed about the risks associated with peanut exposure and the availability of therapeutic options, advanced solutions offer features such as desensitization capabilities, emergency preparedness, and improved quality of life, providing a compelling alternative to constant anxiety and strict avoidance. Furthermore, the growing popularity of allergen-aware environments in schools and public places and the desire for safer food consumption experiences are making Peanut Allergy Treatment an integral component of public health initiatives, offering seamless integration with educational campaigns and emergency response protocols.
- The convenience of improved diagnostic tools, the availability of epinephrine autoinjectors, and the emergence of immunotherapies are key factors propelling the adoption of Peanut Allergy Treatment. The trend towards early intervention and the increasing support from patient advocacy groups further contribute to market growth.
Restraint/Challenge:
“High Cost of Immunotherapies and Risk of Side Effects”
- Concerns regarding the relatively high cost of novel immunotherapies, such as Oral Immunotherapy (OIT) and biologics, coupled with the potential for side effects, including allergic reactions during treatment, pose a significant challenge to broader market penetration
- For instance, a recent study highlighted that the annual cost of some OIT treatments can be substantial, raising anxieties among patients and payers about affordability and accessibility
- Addressing these cost concerns through improved reimbursement policies, patient assistance programs, and demonstrating long-term cost-effectiveness is crucial for encouraging wider adoption. Companies developing immunotherapies emphasize their safety profiles and clinical efficacy in their marketing to reassure both patients and healthcare providers
- Additionally, the need for close medical supervision during the initial phases of OIT and the potential for adverse reactions, although manageable, can be a barrier to treatment initiation for some families. While the potential for transformative patient outcomes exists, the perceived high upfront costs and the need for ongoing medical oversight can still hinder widespread adoption
- Overcoming these challenges through expanded insurance coverage, enhanced patient education on risk management, and developing more convenient and safer administration protocols will be vital for sustained market growth
Peanut Allergy Treatment Market Scope
The market is segmented on the basis of treatment type, age group, distribution channel, and end-user.
- By Drug Type
On the basis of drug type, the market is segmented into Injectable Epinephrine and Antihistamines. The Injectable Epinephrine segment is projected to hold the largest market share. This is due to its critical role as the first-line treatment for severe allergic reactions (anaphylaxis) and its rapid onset of action. The increasing prevalence of allergies worldwide and the rising awareness among individuals about carrying emergency medication contribute to its dominance.
The Antihistamines segment is expected to witness significant growth. While not a substitute for epinephrine in anaphylaxis, antihistamines are widely used for managing milder allergic symptoms, both over-the-counter and prescription. Their broad applicability for various allergic conditions drives consistent demand.
- By Route of Administration
On the basis of route of administration, the market is segmented into Oral and Injectable. The Injectable segment is anticipated to account for the largest market share. This is primarily driven by the immediate and systemic action offered by injectable medications, especially crucial in emergency situations like severe allergic reactions. The need for rapid delivery of active compounds for life-threatening conditions underpins its prevalence.
The Oral segment is expected to exhibit the fastest growth. This is due to the convenience, ease of administration, and non-invasiveness associated with oral medications, making them highly preferred for chronic conditions and self-administration. Advances in drug formulation and patient compliance also contribute to its expanding share.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into Retail Pharmacies, Hospital Pharmacies, and Online Pharmacies. Retail Pharmacies are expected to hold the largest market revenue share. Their widespread accessibility, direct patient interaction, and convenience for prescription refills and over-the-counter purchases make them the primary point of access for many consumers.
Online Pharmacies are anticipated to witness the fastest growth. This is driven by the increasing trend of e-commerce, the convenience of home delivery, competitive pricing, and a wider product selection. The growing consumer preference for digital platforms for healthcare needs further fuels this segment's expansion.
Peanut Allergy Treatment Market Regional Analysis
- North America dominates the Peanut Allergy Treatment Market with the largest revenue share of 40.01% in 2024, driven by a high prevalence of peanut allergies, robust research and development activities, and early regulatory approvals for novel immunotherapies
- The region benefits from a well-established network of allergy specialists, strong patient advocacy groups, and significant healthcare expenditure dedicated to managing severe allergies. This widespread adoption is further supported by proactive public health awareness campaigns and favorable reimbursement policies for approved treatments
U.S. Peanut Allergy Treatment Market Insight
The U.S. Peanut Allergy Treatment Market captured the largest revenue share of 81% within North America in 2024, fueled by the highest incidence of peanut allergy globally, significant investment in allergy research, and the presence of leading pharmaceutical and biotechnology companies developing advanced treatments. The country has been at the forefront of regulatory approvals for OIT and has extensive patient support networks.
Europe Peanut Allergy Treatment Market Insight
The European Peanut Allergy Treatment Market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing awareness, improving diagnostic capabilities, and a rising prevalence of food allergies across several European countries. European regulatory bodies are increasingly streamlining the approval process for innovative allergy therapies. The market also benefits from collaborative research efforts and growing patient advocacy for food allergy management.
U.K. Peanut Allergy Treatment Market Insight
The U.K. Peanut Allergy Treatment Market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing public and healthcare professional awareness, active research initiatives in allergy immunology, and the National Health Service (NHS) focusing on improving access to specialized allergy care. The country's robust clinical trial network also contributes to market growth.
Germany Peanut Allergy Treatment Market Insight
The German Peanut Allergy Treatment Market is expected to expand at a considerable CAGR during the forecast period, fueled by a strong healthcare system, advanced diagnostic capabilities for allergies, and a growing emphasis on personalized medicine. Germany's commitment to clinical research and adoption of evidence-based treatments positions it as a key market for peanut allergy therapies.
Asia-Pacific Peanut Allergy Treatment Market Insight
The Asia-Pacific Peanut Allergy Treatment Market is poised to grow at the fastest CAGR of over 24% in 2024, driven by increasing urbanization, changing dietary habits, rising incidence of food allergies, and improving healthcare infrastructure in countries such as China, Japan, and India. Growing awareness campaigns and increasing access to specialized allergy care contribute to regional growth.
Japan Peanut Allergy Treatment Market Insight
The Japan Peanut Allergy Treatment Market is gaining momentum due to a high level of awareness regarding food allergies, sophisticated diagnostic technologies, and a proactive approach to allergy research and development. The Japanese market benefits from a well-established healthcare system and a focus on integrating innovative treatments into clinical practice.
China Peanut Allergy Treatment Market Insight
The China Peanut Allergy Treatment Market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to its vast population, increasing prevalence of food allergies, and expanding investments in healthcare facilities and allergy research. Government efforts to improve public health awareness and a growing middle class seeking advanced medical solutions are key factors propelling the market in China.
Peanut Allergy Treatment Market Share
The Peanut Allergy Treatment industry is primarily led by well-established companies, including:
- Mylan N.V.
- Sanofi
- Pfizer Inc.
- kaleo, Inc.
- DBV Technologies
- Cambridge Peanut Allergy Clinic
- Regeneron
- ANAPTYSBIO, INC.
- ALK
- Stallergenes Greer
- Astellas Pharma Inc.
- Immunomic Therapeutics, Inc
- HAL Allergy B.V.
- Aravax
- Aimmune Therapeutics
- Prota Therapeutics Pty Ltd
Latest Developments in Global Peanut Allergy Treatment Market
- In April 2023, Aimmune Therapeutics, Inc. (Nestle Health Science), a leading company in food allergy treatments, announced positive real-world data from its oral immunotherapy product, demonstrating sustained desensitization in a larger patient population than clinical trials. This data supports the long-term effectiveness of their therapy
- In March 2023, DBV Technologies, a clinical-stage biopharmaceutical company, reported progress in its Phase 3 clinical trial for a novel epicutaneous (patch) immunotherapy for peanut allergy, signaling potential for a non-oral treatment option. This advancement aims to provide an alternative for patients unable to tolerate oral therapies
- In March 2023, Sanofi and Regeneron Pharmaceuticals, Inc., collaborating partners, announced the initiation of a new Phase 3 study investigating their biologic drug (Omalizumab) for broader use in multiple food allergies, including peanut, following previous successes in chronic spontaneous urticaria and asthma. This expansion broadens the potential application of their existing drug
- In February 2023, Kaléo, Inc., a privately held pharmaceutical company, introduced an updated version of its epinephrine autoinjector with enhanced user features and connectivity options to improve patient compliance and emergency response. This innovation aims to make emergency treatment more accessible and user-friendly
- In January 2023, Camallergy, a U.K.-based biopharmaceutical company, presented preclinical data for a new rapid oral immunotherapy formulation for peanut allergy, demonstrating promising results in accelerating desensitization with fewer treatment sessions. This development could lead to faster and more convenient treatment regimens
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.