Global Pediatric Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
166.87 Billion
USD
428.15 Billion
2024
2032
USD
166.87 Billion
USD
428.15 Billion
2024
2032
| 2025 –2032 | |
| USD 166.87 Billion | |
| USD 428.15 Billion | |
|
|
|
|
Pediatric Drugs Market Size
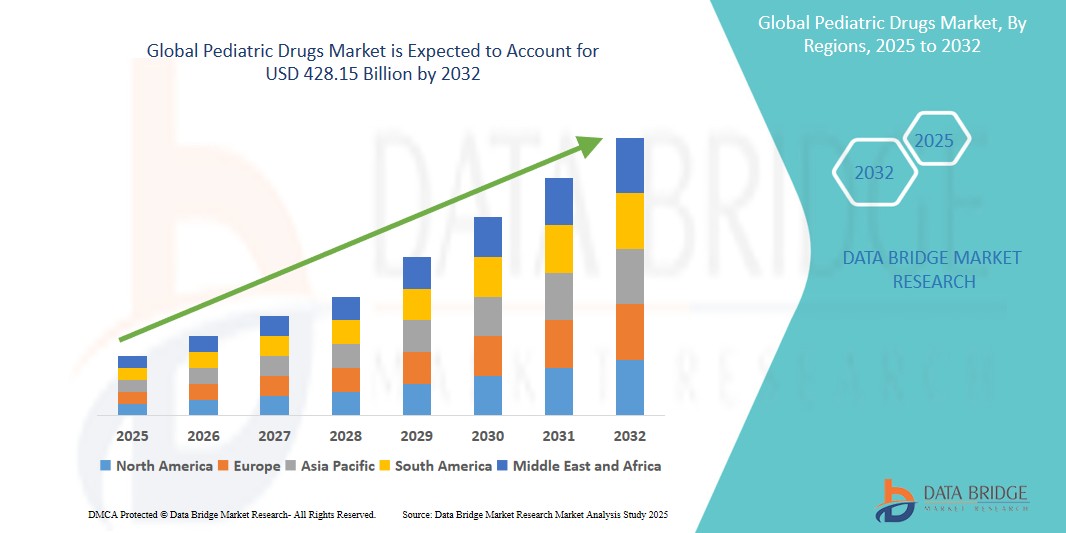
- The global pediatric drugs market was valued at USD 166.87 billion in 2024 and is expected to reach USD 428.15 billion by 2032
- During the forecast period of 2025 to 2032 the market is such as to grow at a CAGR of 12.50%, primarily driven by the increasing demand for pediatric drugs for treating various health conditions in children
- This growth is driven by factors such as the rising awareness of pediatric healthcare needs and the development of specialized pediatric formulations and treatments
Pediatric Drugs Market Analysis
- The pediatric drugs market is experiencing steady growth due to the increasing demand for specialized medications designed for children’s unique health needs
- The market is seeing a shift toward developing pediatric formulations of existing drugs, with a focus on improving dosage forms, flavors, and ease of administration for children
- New product innovations are contributing to the market’s expansion, including the development of oral syrups, chewable tablets, and injectable medications that are more suitable for pediatric patients
- The growing prevalence of chronic diseases in children, such as asthma, diabetes, and epilepsy, is also driving the demand for pediatric drugs tailored to managing these conditions
- For instance, medications such as liquid formulations of diabetes drugs and child-friendly versions of antibiotics are becoming more widely used as they offer improved compliance and better treatment outcomes for young patients
- In conclusion, the market is expected to continue growing as the focus on pediatric healthcare becomes more prominent, with innovations in drug formulations and treatment options for children at the forefront of market advancements
Report Scope and Pediatric Drugs Market Segmentation
|
Attributes |
Pediatric Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Pediatric Drugs Market Trends
“The Increase of Advancements in Pediatric Drug Development”
- The pediatric drugs market is experiencing significant growth, attributed to the increasing prevalence of chronic diseases among children and advancements in drug formulations tailored for pediatric care
- Pharmaceutical companies are focusing on developing medications specifically designed for children, enhancing treatment adherence and safety
- Regulatory bodies are implementing initiatives to encourage research and development in pediatric pharmacology, aiming to address the unique therapeutic needs of children
- There is a growing trend towards personalized medicine in pediatrics, utilizing genetic and biomarker information to tailor treatments for individual pediatric patients
- For instance, the U.S. Food and Drug Administration expanded the approval of Avadel Pharmaceuticals' sleep disorder drug Lumryz to children aged 7 and older, highlighting the industry's commitment to addressing pediatric health needs
- These trends underscore the dynamic nature of the pediatric drugs market, reflecting a concerted effort to enhance therapeutic options and outcomes for children worldwide
Pediatric Drugs Market Dynamics
Driver
“Rising Burden of Pediatric Diseases”
- The rise in chronic pediatric conditions such as asthma, diabetes, epilepsy, and congenital disorders is driving the demand for specialized pediatric drugs
- Children react differently to medications, requiring age-specific drug formulations that are safe, effective, and easy to administer
- Pediatric medications often come in forms such as syrups, chewable tablets, and dissolvable strips to ensure higher compliance and minimize side effects
- Increasing awareness among parents and healthcare providers about early diagnosis and treatment of childhood diseases is further boosting the demand for pediatric drugs
- For instance, childhood immunization and public health campaigns are enhancing access to pediatric care and medications
- The growing need for tailored treatments continues to drive market growth and foster innovation in pediatric drug development
Opportunity
“Focus on Pediatric-Specific Drug Development”
- The pediatric drugs market is experiencing a significant opportunity as more drugs are being developed specifically for children, addressing the limitations of adult-adapted treatments
- Regulatory authorities and healthcare organizations are encouraging pediatric clinical trials, which has opened new avenues for innovation in pediatric oncology, infectious diseases, and rare genetic disorders
- Pharmaceutical companies are focusing on developing child-friendly dosage forms such as orally disintegrating tablets, flavored syrups, and chewables to enhance ease of administration and patient adherence
- The rise of personalized medicine, considering factors such as age, weight, and genetic variations, is leading to more precise and effective treatments for children
- For instance, the development of pediatric oncology drugs specifically designed for younger patients is gaining momentum, addressing previously unmet needs
- This shift towards specialized treatments offers a major growth opportunity for the pediatric drugs market, improving the safety and efficacy of therapies for children worldwide
Restraint/Challenge
“Ethical and Logistical Barriers in Pediatric Trials”
- One of the biggest challenges in the pediatric drugs market is the complexity of conducting clinical trials involving children, which face ethical concerns and strict regulatory scrutiny
- Parents are often cautious about enrolling their children in clinical trials, especially when it involves experimental drugs or invasive treatments, adding an additional layer of complexity to the process
- Pediatric trials are scientifically challenging due to variations in growth, metabolism, and development at different childhood stages, making it necessary to adjust dosage and safety protocols for specific age groups
- Recruitment and retention of pediatric participants are often lower, limiting the size and quality of studies, while smaller sample sizes can delay drug approvals and discourage investment in pediatric research
- For instance, the limited number of pediatric clinical trials for rare genetic disorders slows down the availability of tailored treatments for these children
- These hurdles contribute to slower drug development timelines and hinder timely access to innovative therapies for children, requiring collaborative efforts to overcome them
Pediatric Drugs Market Scope
The market is segmented on the basis of drug type, route of administration, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Drug Type |
|
|
By Route of Administration |
|
|
By End User |
|
|
By Distribution Channel |
|
Pediatric Drugs Market Regional Analysis
“North America is the Dominant Region in the Pediatric Drugs Market”
- North America held the largest share of the pediatric drugs market in 2023, accounting for approximately 45% of the global market
- The region's dominance is attributed to its advanced healthcare infrastructure, high healthcare expenditure, and a significant pediatric population requiring specialized medications
- U. S. is expected to dominate the market due to its advanced healthcare infrastructure, high R&D investment, and strong regulatory support from the FDA for pediatric drug development
- Regulatory support from agencies such as the U.S. Food and Drug Administration has facilitated the approval and availability of pediatric drugs, ensuring timely access to safe and effective therapies
- The presence of leading pharmaceutical companies and ongoing research and development activities contribute to the continuous growth and innovation within the pediatric drugs sector in North America
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is witnessing rapid growth in the pediatric drugs market due to the increasing demand for specialized medications for children
- This growth is driven by countries such as China, India, Japan, Australia, and South Korea, which collectively contribute to a substantial pediatric population
- China is expected to be fastest growing in the region due to its large pediatric population, rapid healthcare advancements, and growing demand for child-specific medications
- Improvements in healthcare infrastructure, rising awareness about pediatric health, and better access to advanced medications are fueling market expansion in this region
- Economic development has led to increased affordability and demand for specialized pediatric treatments
Pediatric Drugs Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Viatris Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd.(Israel)
- Sanofi (France)
- Pfizer Inc. (US)
- GlaxoSmithKline plc (UK)
- Novartis AG (Switzerland)
- Zydus Cadila (India)
- AstraZeneca (UK)
- Johnson & Johnson (US)
- Bayer AG (Germany)
- Sun Pharmaceutical Industries Ltd. (India)
- Bristol-Myers Squibb Company (US)
- Eli Lilly and Company (US)
- Cipla Inc. (US)
- Mallinckrodt (US)
- Apotex Inc. (Canada)
- Hikma Pharmaceuticals PLC (UK)
- Endo International plc (Ireland)
Latest Developments in Global Pediatric Drugs Market
- In February 2025, the World Health Organization and St. Jude Children’s Research Hospital initiated the Global Platform for Access to Childhood Cancer Medicines, aiming to provide quality-assured cancer treatments to pediatric patients in low- and middle-income countries. The platform began distributing medications in Mongolia and Uzbekistan, with plans to expand to Ecuador, Jordan, Nepal, and Zambia. The initiative seeks to reach 50 countries over the next 5 to 7 years, ultimately aiming to treat approximately 120,000 children with cancer annually, thereby significantly reducing mortality rates in these regions
- In July 2024, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) launched the Pediatric and Rare Disease Drug Consultation Center to support the development of drugs for pediatric (obesity) and rare diseases(cancer, etc). This initiative aims to address the drug lag in these critical areas and offers consultation services for the simultaneous development of adult and pediatric drugs, as encouraged by Japan’s Ministry of Health, Labour and Welfare (MHLW)
- In November 2024, the World Health Organization (WHO) emphasized the importance of improving access to better pediatric medicines on World Children’s Day. The initiative aims to address the lack of suitable medications for children, especially in low- and middle-income countries. WHO calls for more research and development to create child-specific treatments and improve the overall health and well-being of children worldwide
- In 2024, the FDA approved several significant treatments for pediatric care. One notable approval was for Dupilumab (Dupixent), making it the first treatment specifically indicated for children with eosinophilic esophagitis. Another key approval was for Epinephrine nasal spray (Neffy), marking it as the first nasal spray approved for treating anaphylaxis in both adults and pediatric patients aged 7 years and older
- In April 2024, the U.S. Food and Drug Administration approved Lutathera (lutetium Lu 177 dotatate) for treating pediatric patients aged 12 and older with somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including those in the foregut, midgut, and hindgut. This approval marks Lutathera as the first therapy specifically reviewed and approved for pediatric patients with GEP-NETs. The decision was based on the NETTER-P trial, which demonstrated that Lutathera's safety profile in pediatric patients was consistent with that observed in adults
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












