Global Pediatric Neuroblastoma Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.21 Billion
USD
2.55 Billion
2024
2032
USD
1.21 Billion
USD
2.55 Billion
2024
2032
| 2025 –2032 | |
| USD 1.21 Billion | |
| USD 2.55 Billion | |
|
|
|
|
Pediatric Neuroblastoma Treatment Market Analysis
The global pediatric neuroblastoma treatment market is driven by the increasing incidence of neuroblastoma in children, which remains one of the most common cancers affecting children under the age of 5. Neuroblastoma accounts for approximately 6-10% of all childhood cancers, with around 650 new cases diagnosed annually in the U.S. alone. The disease is predominantly diagnosed in children younger than 5, and while it can arise in various parts of the body, it most often starts in the adrenal glands. The survival rate for localized neuroblastoma is high, but for advanced or metastatic cases, the prognosis remains challenging, with survival rates dropping significantly. Advances in treatment options, including chemotherapy, immunotherapy, and targeted therapies, are continuously improving outcomes, although the need for more effective and less toxic treatments remains critical. Additionally, the increasing prevalence of relapsed and refractory neuroblastoma highlights the demand for innovative therapies that can offer better long-term survival and quality of life for pediatric patients. Ongoing clinical trials and breakthroughs in precision medicine are expected to play a crucial role in the future treatment environment.
Pediatric Neuroblastoma Treatment Market Size
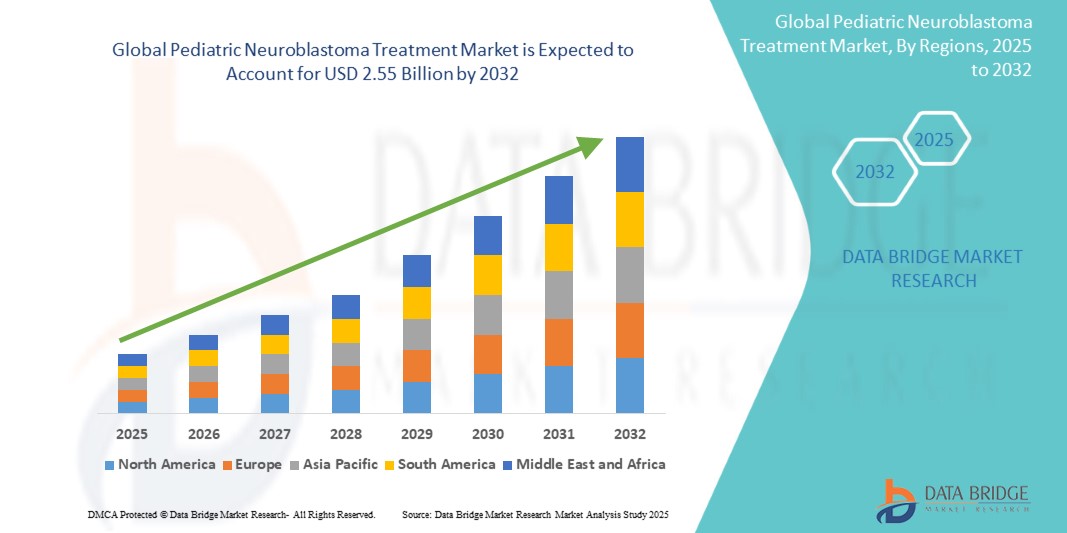
Global pediatric neuroblastoma treatment market size was valued at USD 1.21 billion in 2024 and is projected to reach USD 2.55 billion by 2032, with a CAGR of 9.40% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Pediatric Neuroblastoma Treatment Market Trends
“Advancements in Early Diagnosis”
Advancements in Early Diagnosis of pediatric neuroblastoma are becoming a significant trend, marked by the development of more precise and efficient diagnostic techniques. Advanced imaging technologies, such as high-resolution ultrasound, MRI, and PET scans, are improving the ability to detect tumors at earlier stages. Additionally, the identification of specific biomarkers in blood and urine is facilitating non-invasive diagnostic methods, enabling earlier detection of neuroblastoma. The trend also includes innovations in genetic testing, where molecular profiling of tumors helps identify mutations or specific characteristics, contributing to more accurate diagnoses. Early diagnosis is increasingly recognized for its role in improving treatment outcomes, as identifying neuroblastoma at an earlier stage allows for more effective intervention and higher survival rates.
Report Scope and Pediatric Neuroblastoma Treatment Market Segmentation
|
Attributes |
Pediatric Neuroblastoma Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
F. Hoffmann-La Roche Ltd (Switzerland), Bristol-Myers Squibb Company (U.S.), Johnson & Johnson Services, Inc. (U.S.), Novartis AG (Switzerland), Eli Lilly and Company (U.S.), Amgen Inc. (U.S.), Merck & Co., Inc. (U.S.), Pfizer Inc. (U.S.), Bayer AG (Germany), Sanofi S.A. (France), AbbVie Inc. (U.S.), GSK plc. (UK), Astellas Pharma Inc. (Japan), Regeneron Pharmaceuticals, Inc. (U.S.), NantKwest, Inc. (U.S.), Ionis Pharmaceuticals, Inc. (U.S.) among others. |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Global Pediatric Neuroblastoma Treatment Market Definition
Pediatric Neuroblastoma Treatment refers to the medical interventions used to manage and cure neuroblastoma, a type of cancer that commonly affects children, particularly those under the age of 5. It involves a combination of therapies designed to eliminate the cancerous cells and reduce the tumor's size or spread. Treatment options may include chemotherapy, radiation therapy, surgery, immunotherapy, targeted therapy, and stem cell transplantation. The choice of treatment depends on factors such as the stage of the disease, the child's age, and overall health. Early detection and aggressive treatment are crucial to improving the prognosis and survival rates for pediatric patients with neuroblastoma.
Pediatric Neuroblastoma Treatment Market Dynamics
Drivers
- Increasing Incidence of Pediatric Neuroblastoma
The increasing incidence of pediatric neuroblastoma is a key factor driving the growth of the global pediatric neuroblastoma treatment market. Neuroblastoma is one of the most common cancers in children, particularly affecting those under the age of 5. With approximately 650 new cases diagnosed annually in the U.S. alone, the rising number of diagnoses worldwide is putting pressure on healthcare systems to develop more effective treatment options. As the disease can occur in various forms, ranging from localized to metastatic, it requires specialized therapies to address its diverse stages. This growing incidence not only underscores the need for better treatment methods but also stimulates investment in research and clinical trials focused on improving therapeutic outcomes. The increasing awareness and diagnosis of neuroblastoma, combined with advancements in early detection and treatment, are expected to continue fueling the demand for innovative therapies and contributing to the expansion of the pediatric neuroblastoma treatment market. For instance, in August 2024, according to an article published by RedHill Biopharma Ltd., neuroblastoma is a rare cancer, yet it is the most common malignancy in infants, with a median age of diagnosis at 17 months. In the U.S., it represents approximately 10% of all childhood cancer cases and 15% of pediatric cancer-related deaths. The high prevalence and mortality rate of neuroblastoma in children are expected to drive demand for improved treatments, fueling market growth as researchers and healthcare providers focus on developing more effective therapies and early detection methods.
- Advancements in Immunotherapy and Targeted Therapies
Advancements in immunotherapy and targeted therapies are significantly driving the growth of the global pediatric neuroblastoma treatment market. Immunotherapies, such as monoclonal antibodies and immune checkpoint inhibitors, have emerged as highly promising treatments. These therapies work by stimulating the immune system to better recognize and attack cancer cells, offering a more targeted approach compared to traditional chemotherapy, which can affect healthy cells and cause significant side effects. Targeted therapies, which aim to attack specific molecules involved in cancer cell growth, are also gaining traction. These innovations are proving to be more effective, with fewer side effects, leading to improved patient outcomes, especially in high-risk cases or relapsed neuroblastoma. As a result, the increased availability and application of these advanced treatments are expanding the market, offering new hope for pediatric patients and improving long-term survival rates. This progress in treatment modalities is a key driver of continued growth in the pediatric neuroblastoma treatment market. In December 2024, according to an article published by ScienceDirect, recent advancements in cancer immunotherapy, including immune checkpoint inhibitors (ICIs) and CAR-T cell therapy, have greatly improved cancer treatment. ICIs, such as PD-1/PD-L1 and CTLA-4 inhibitors, boost the immune response against tumors, while CAR-T therapy shows significant success in treating hematologic cancers. These innovations are expected to drive the Global Pediatric Neuroblastoma Treatment Market by paving the way for targeted immunotherapies, offering potential breakthroughs in treating neuroblastoma and similar cancers.
Opportunities
- Development of Personalized Medicine
The development of personalized medicine presents a key opportunity in the global pediatric neuroblastoma treatment market. By utilizing genetic profiling and molecular diagnostics, healthcare providers can tailor treatment plans to the specific genetic and molecular characteristics of each patient’s tumor. This customization ensures more effective targeting of cancer cells while minimizing damage to healthy tissues, thus reducing side effects often seen with traditional therapies. Personalized medicine is particularly beneficial for high-risk or relapsed neuroblastoma cases, as it offers a more precise approach to treatment, increasing the chances of successful outcomes. Advances in molecular biology, such as identifying tumor-specific biomarkers and mutations, are expanding the potential for targeted therapies. This precision-focused approach not only enhances the effectiveness of existing treatments but also paves the way for the development of new drugs designed to treat neuroblastoma more efficiently, ultimately improving survival rates and quality of life for pediatric patients. For instance, in January 2024, according to an article published by NCBI, precision medicine focuses on tailoring treatments based on individual patient and tumor characteristics to enhance efficacy and minimize toxicity, offering new hope for neuroblastoma (NB) patients. This approach presents a significant opportunity for the Global Pediatric Neuroblastoma Treatment Market, as it drives the development of personalized therapies with improved outcomes.
- Increased Research and Clinical Trials
The expansion of clinical research and trials targeting pediatric neuroblastoma offers a significant opportunity for market growth. As pharmaceutical companies and research institutions intensify efforts to develop novel therapies, the treatment landscape for neuroblastoma is evolving rapidly. Ongoing clinical trials focused on innovative drug formulations, gene therapies, and advanced molecular targets are paving the way for new, more effective treatments. These trials not only offer hope for improved outcomes in pediatric patients but also increase the likelihood of new drug approvals and updated treatment protocols, which are essential for tackling high-risk or relapsed neuroblastoma cases. The exploration of cutting-edge therapies such as immunotherapies, targeted therapies, and gene editing techniques holds promise for providing more effective and personalized treatments. These advancements contribute to both market innovation and better patient outcomes, driving growth in the global pediatric neuroblastoma treatment market and improving survival rates for affected children. For instance, in April 2024, according to an article published by The Institute of Cancer Research, in early 2023, a clinical trial revealed that lorlatinib, initially developed for adult lung cancer, showed promise in treating neuroblastoma in children with a specific ALK mutation. This presents an opportunity for further research and clinical trials, supporting the growth of innovative therapies and highlighting the industry's increasing focus on developing targeted treatments for pediatric cancers.
Restraints/Challenges
- High Cost of Neuroblastoma Treatment
The high cost of treatment is a major restraint in the global pediatric neuroblastoma treatment market. Many of the therapies used to treat neuroblastoma, including advanced immunotherapies, stem cell transplants, and targeted therapies, come with significant price tags. These treatments often require prolonged therapy regimens, which further increases the financial burden on families and healthcare systems. For families, especially in low- and middle-income countries, the cost can be prohibitive, limiting access to potentially life-saving treatments. The high cost of such therapies also presents challenges in reimbursement policies, with some patients unable to secure coverage or funding for their treatments. This limits the adoption of these therapies on a broader scale and impedes market growth. As a result, the financial barriers to accessing advanced treatments pose a significant challenge, making it difficult to ensure equitable access to the best possible care for children with neuroblastoma across different regions.
- Limited Treatment Options for Relapsed Neuroblastoma
Limited treatment options for relapsed neuroblastoma represent a significant challenge in the global pediatric neuroblastoma treatment market. Neuroblastoma, especially in its high-risk or advanced stages, has a high rate of relapse, even after aggressive therapies such as chemotherapy, radiation, and stem cell transplants. Once relapsed, neuroblastoma is more difficult to treat, with fewer effective therapeutic options available. The cancer may become resistant to conventional treatments, reducing the chances of achieving remission. Additionally, many of the available options for relapsed cases are still in clinical trials or are considered experimental, which introduces uncertainties around their efficacy and safety. The lack of proven, standardized treatments for relapsed neuroblastoma continues to negatively impact survival rates and complicates treatment plans. This challenge emphasizes the need for ongoing research and innovation to develop more effective therapies for relapsed neuroblastoma, ultimately improving patient outcomes and survival rates for those facing this difficult form of cancer. For instance, in July 2024, according to an article published by Taylor & Francis Online, neuroblastoma (NB) continues to be a difficult pediatric cancer to treat, especially for high-risk cases, with limited available treatment options. This presents a major challenge for the Global Pediatric Neuroblastoma Treatment Market, as the lack of effective therapies for high-risk patients hinders market growth and therapeutic advancements.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Pediatric Neuroblastoma Treatment Market Scope
The market is segmented on the basis of treatment type, drug type, diagnosis type, age group, therapy mode, and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Treatment Type
- Chemotherapy
- Radiation Therapy
- Surgery
- Immunotherapy
- Stem Cell Transplantation
- Targeted Therapy
Drug Type
- Alkylating Agents
- Monoclonal Antibodies
- Cytotoxic Drugs
- Biologic Therapy Drugs
Diagnosis Type
- Imaging
- Biopsy
- Blood Tests
- Bone Marrow Aspiration
Age Group
- Infants (0-1 year)
- Toddlers (1-3 years)
- Children (4-12 years)
- Adolescents (13-18 years)
Therapy Mode
- Monotherapy
- Combination Therapy
End-User
- Hospitals
- Cancer Treatment Centers
- Ambulatory Surgical Centers
Pediatric Neuroblastoma Treatment Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, treatment type, drug type, diagnosis type, age group, therapy mode, and end-user as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to its advanced healthcare infrastructure, which provides access to state-of-the-art diagnostic and therapeutic facilities. These advanced systems enable early detection, precise diagnosis, and comprehensive management of neuroblastoma, contributing to improved patient outcomes. High healthcare spending in the region further supports the adoption of innovative and high-cost therapies, including targeted treatments and immunotherapies. The financial capability of healthcare systems in North America allows for the integration of cutting-edge technologies and therapies, making the region a leader in pediatric neuroblastoma care.
Asia-Pacific is expected to be the fastest growing due to several transformative factors. The increasing healthcare investments across emerging economies such as China, India, and Southeast Asian nations are driving significant improvements in medical infrastructure and oncology care. Governments and private sectors are channeling funds into establishing specialized cancer treatment centers, acquiring advanced medical technologies, and enhancing the overall capacity of healthcare systems to manage complex diseases such as neuroblastoma.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Pediatric Neuroblastoma Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Pediatric Neuroblastoma Treatment Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Novartis AG (Switzerland)
- Eli Lilly and Company (U.S.)
- Amgen Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Bayer AG (Germany)
- Sanofi S.A. (France)
- AbbVie Inc. (U.S.)
- GSK plc. (UK)
- Astellas Pharma Inc. (Japan)
- Regeneron Pharmaceuticals, Inc. (U.S.)
- NantKwest, Inc. (U.S.)
- Ionis Pharmaceuticals, Inc. (U.S.)
Latest Developments in Pediatric Neuroblastoma Treatment Market
- In August 2024, RedHill Biopharma Ltd. announced that the U.S. Food and Drug Administration (FDA) has granted orphan-drug designation to opaganib for the treatment of neuroblastoma, a childhood cancer that originates from immature nerve cells and is responsible for 15% of pediatric cancer-related deaths. This designation will help the company by providing regulatory benefits such as market exclusivity, tax incentives, and reduced development costs, accelerating the potential for opaganib to reach the market and offer new treatment options for pediatric neuroblastoma patients
- In August 2024, the FDA has granted both rare pediatric disease designation and orphan drug designation to INV724 for treating neuroblastoma patients. These designations will benefit the company by providing regulatory advantages such as market exclusivity, financial incentives, and expedited development processes, helping accelerate the treatment's path to market
- In February 2024, NBUK and SKCUK, two of the U.K.'s largest funders of neuroblastoma research, have formed a new partnership with a clear plan to work more closely and regularly together to improve outcomes for children with the condition of neuroblastoma. This collaboration will help the company by increasing research funding, fostering innovation, and accelerating the development of effective treatments.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.