Global Pegylated Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
16.01 Billion
USD
24.53 Billion
2025
2033
USD
16.01 Billion
USD
24.53 Billion
2025
2033
| 2026 –2033 | |
| USD 16.01 Billion | |
| USD 24.53 Billion | |
|
|
|
|
PEGylated Drugs Market Size
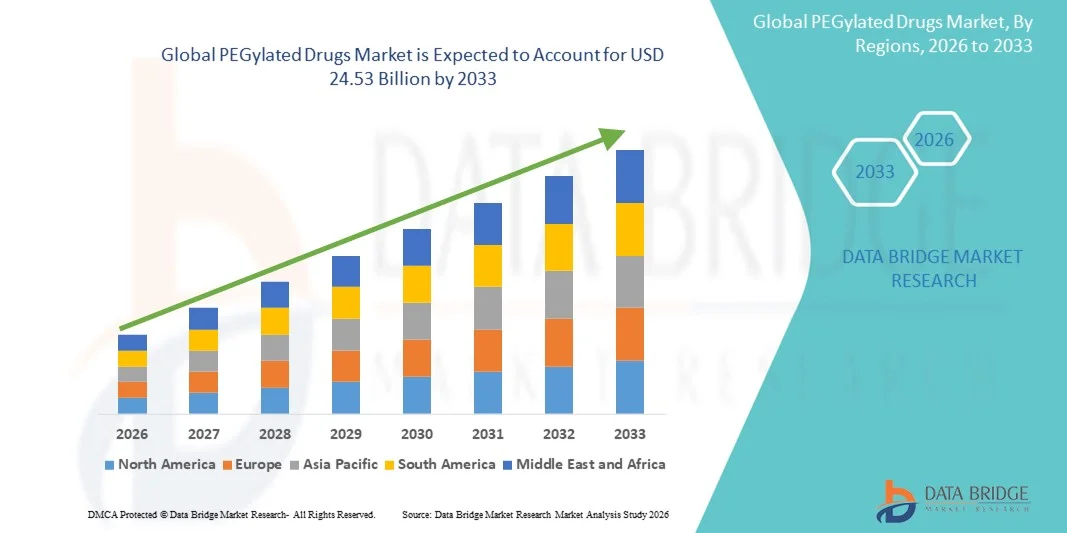
- The global PEGylated drugs market size was valued at USD 16.01 billion in 2025 and is expected to reach USD 24.53 billion by 2033, at a CAGR of 5.48% during the forecast period
- The market growth is largely fueled by increasing adoption of biologics and biopharmaceuticals, along with technological advancements in PEGylation techniques that enhance drug stability, efficacy, and half-life
- Furthermore, rising demand for targeted therapies and improved patient compliance is driving the integration of PEGylated drugs across therapeutic areas, including oncology, hematology, and chronic diseases. These converging factors are accelerating the uptake of PEGylated drug solutions, thereby significantly boosting the industry's growth
PEGylated Drugs Market Analysis
- PEGylated drugs, involving the attachment of polyethylene glycol (PEG) to therapeutic molecules, are increasingly vital components of modern biopharmaceuticals due to their enhanced stability, prolonged half-life, and reduced immunogenicity, making them essential in chronic disease management and targeted therapies
- The escalating demand for PEGylated drugs is primarily fueled by the rising prevalence of chronic and rare diseases, growing adoption of biologics, and the need for improved patient compliance through less frequent dosing schedules
- North America dominated the PEGylated drugs market with the largest revenue share of 43.2% in 2025, characterized by advanced healthcare infrastructure, high R&D investment, and a strong presence of leading pharmaceutical companies, with the U.S. experiencing substantial growth driven by innovations in PEGylated interferons and colony-stimulating factors and increasing regulatory approvals
- Asia-Pacific is expected to be the fastest growing region in the PEGylated drugs market during the forecast period due to expanding healthcare access, rising healthcare expenditure, and growing biopharmaceutical manufacturing capabilities
- Interferons segment dominated the PEGylated drugs market with a market share of 38.3% in 2025, driven by their extensive use in treating hepatitis and multiple sclerosis and the continuous development of long-acting PEGylated formulations improving patient adherence
Report Scope and PEGylated Drugs Market Segmentation
|
Attributes |
PEGylated Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
PEGylated Drugs Market Trends
Advancements in Long-Acting and Targeted Therapies
- A significant and accelerating trend in the global PEGylated drugs market is the development of long-acting formulations that improve therapeutic efficacy and patient adherence, particularly in chronic and rare disease treatments
- For instance, PEGylated interferons with extended half-lives allow less frequent dosing for hepatitis patients, reducing the treatment burden and enhancing compliance
- Innovations in targeted PEGylated therapies are enabling selective drug delivery, minimizing off-target effects, and improving clinical outcomes, thereby increasing the attractiveness of PEGylated biologics for physicians and patients
- The integration of PEGylation with advanced biopharmaceutical platforms facilitates the development of multifunctional therapeutics, combining extended half-life, reduced immunogenicity, and enhanced bioavailability in a single molecule
- Growing collaborations between pharmaceutical companies and research institutes are accelerating the development of next-generation PEGylated drugs with enhanced safety and efficacy profiles
- Advances in personalized medicine are driving the use of PEGylated drugs tailored to patient-specific needs, optimizing therapeutic outcomes and minimizing adverse effects
- This trend towards highly effective, patient-friendly, and precision-oriented PEGylated drugs is driving R&D investments and shaping competitive strategies in the biopharmaceutical industry
- The demand for PEGylated drugs with improved pharmacokinetic profiles is growing rapidly across oncology, autoimmune, and rare disease segments, as healthcare providers and patients increasingly prioritize convenience, efficacy, and safety
PEGylated Drugs Market Dynamics
Driver
Increasing Prevalence of Chronic and Rare Diseases
- The rising incidence of chronic and rare diseases, coupled with the growing adoption of biologics, is a significant driver for the heightened demand for PEGylated drugs
- For instance, PEGylated colony-stimulating factors are increasingly used in oncology to reduce neutropenia-related complications during chemotherapy, driving market growth
- As healthcare systems and patients seek therapies that reduce dosing frequency while maintaining efficacy, PEGylated drugs offer a compelling solution through extended half-life and improved bioavailability
- Furthermore, the rising awareness of advanced treatment options and patient preference for convenient dosing schedules are accelerating the adoption of PEGylated therapeutics
- The expansion of biopharmaceutical R&D and the increasing number of PEGylated drug approvals from regulatory authorities are further reinforcing market growth
- Companies focusing on innovative PEGylation techniques and pipeline expansion are contributing to the increasing uptake of PEGylated drugs in both developed and emerging markets
- Increasing government initiatives and funding for biologics and PEGylated drug research are supporting market expansion and accessibility
- Rising collaboration between pharmaceutical companies and contract development and manufacturing organizations (CDMOs) is enabling faster commercialization of PEGylated therapeutics globally
Restraint/Challenge
High Costs and Regulatory Hurdles
- The relatively high development and manufacturing costs of PEGylated drugs, along with stringent regulatory requirements, pose a significant challenge to broader market penetration
- For instance, complex PEGylation processes and extended clinical trials increase the cost of production and time-to-market, limiting accessibility in cost-sensitive regions
- Ensuring consistent quality, safety, and efficacy in PEGylated formulations is critical, as regulatory authorities impose strict guidelines for approval, which can delay commercialization
- In addition, potential immunogenicity or adverse reactions associated with PEGylated drugs require careful monitoring, adding to development complexity and market entry barriers
- While biosimilar PEGylated drugs are emerging, the perception of high costs and regulatory scrutiny can still hinder adoption in certain markets or patient populations
- Overcoming these challenges through cost optimization, streamlined regulatory pathways, and enhanced patient education will be vital for sustained market growth
- Limited awareness among healthcare providers and patients about the benefits of PEGylated drugs in emerging markets can slow adoption rates despite their clinical advantages
- Supply chain complexities, including the need for specialized manufacturing facilities and cold-chain logistics, can pose additional hurdles to timely and widespread distribution
PEGylated Drugs Market Scope
The market is segmented on the basis of molecule, type, disease indication, application, and sales channel.
- By Molecule
On the basis of molecule, the PEGylated drugs market is segmented into protein, FAB’ fragment, enzyme, and aptamer. The protein segment dominated the market with the largest revenue share in 2025, driven by the widespread use of PEGylated proteins in therapeutics such as interferons, enzymes, and hormones. Proteins are favored for PEGylation due to their high therapeutic value, long half-life extension, and reduced immunogenicity. The segment benefits from established PEGylation technologies and high clinical acceptance across chronic and rare disease treatments. Healthcare providers and patients prefer protein-based PEGylated drugs for predictable pharmacokinetics and safety profiles. Regulatory approvals and reimbursement support further reinforce the segment’s market leadership. Strong R&D pipelines targeting protein therapeutics continue to drive the dominance of this segment.
The FAB’ fragment segment is expected to witness the fastest growth from 2026 to 2033, driven by rising demand for targeted therapies in oncology and autoimmune diseases. FAB’ fragments offer high specificity and low immunogenicity, making them ideal candidates for PEGylation to improve stability and circulation time. The segment benefits from personalized medicine approaches where conventional biologics face limitations. Increasing clinical trials and R&D investments for PEGylated FAB’ fragments are fueling growth. Pharmaceutical collaborations for novel FAB’ therapies are also accelerating commercialization. The rising focus on innovative drug delivery mechanisms further contributes to this segment’s rapid adoption.
- By Type
On the basis of type, the market is segmented into interferons, colony-stimulating factors (CSFs), monoclonal antibodies (mAbs), and other types. The interferons segment dominated the market in 2025 with a market share of 38.3% due to extensive use in treating hepatitis and multiple sclerosis, coupled with advantages such as reduced dosing frequency and improved patient adherence. PEGylated interferons are a standard of care in many chronic conditions. The segment benefits from innovations in long-acting formulations that enhance pharmacokinetics and minimize side effects. Strong clinician trust and inclusion in treatment guidelines reinforce their market leadership. High patient awareness and government programs for hepatitis management further boost adoption. The availability of multiple approved PEGylated interferon products supports consistent market demand.
The monoclonal antibodies (mAbs) segment is expected to register the fastest growth during the forecast period, fueled by increasing use in cancer immunotherapy and autoimmune disease treatments. PEGylation improves mAb half-life and reduces immunogenicity, enhancing therapeutic effectiveness. Rising oncology R&D investments and regulatory approvals are driving market expansion. Personalized medicine and targeted therapy strategies increase the appeal of PEGylated mAbs. The segment also benefits from partnerships between biotech firms and large pharmaceutical companies. Clinical trial pipelines for novel PEGylated mAbs further support rapid growth.
- By Disease Indication
On the basis of disease indication, the market is segmented into gastrointestinal disorders, cancer, multiple sclerosis, hepatitis, and other disease indications. The hepatitis segment dominated the market in 2025, driven by PEGylated interferons as a first-line treatment and proven efficacy in viral suppression. Government initiatives and screening programs increase therapy adoption. Reduced injection frequency improves patient adherence and overall treatment outcomes. The segment benefits from strong physician preference and established treatment guidelines. Regulatory approvals for multiple PEGylated interferons reinforce market dominance. The segment’s growth is further supported by high awareness programs targeting hepatitis patients.
The cancer segment is anticipated to witness the fastest growth from 2026 to 2033, due to rising cancer incidence and adoption of PEGylated biologics for targeted therapy. PEGylation improves therapeutic index and minimizes systemic toxicity, which is critical in oncology. Expanding clinical trial pipelines and oncology-focused R&D support growth. Patient preference for safer, long-acting therapies drives adoption. Government and private funding for cancer treatment further boost the segment. The segment also benefits from advanced PEGylation techniques improving drug stability and bioavailability.
- By Application
On the basis of application, the market is segmented into cancer, autoimmune disease, hepatitis, multiple sclerosis, hemophilia, gastrointestinal disorder, and others. The hepatitis application segment dominated the market in 2025 due to widespread use of PEGylated interferons in chronic hepatitis therapy. Long-acting formulations reduce dosing frequency, improving compliance. Strong adoption in North America and Europe supports revenue growth. Government and NGO programs promoting hepatitis treatment further enhance the segment. PEGylated drugs’ clinical efficacy and safety reinforce physician preference. High patient awareness and reimbursement coverage maintain strong market penetration.
The cancer application segment is expected to record the fastest growth during the forecast period, fueled by PEGylated therapeutics that selectively target tumor cells while minimizing off-target effects. Rising cancer prevalence and approvals of new PEGylated oncology drugs accelerate adoption. Patients prefer long-acting therapies with improved safety profiles. Clinical trials exploring combination therapies expand the potential use cases. Strong investments in oncology-focused PEGylated biologics support growth. Personalized medicine initiatives further enhance the segment’s demand and market expansion.
- By Sales Channel
On the basis of sales channel, the market is segmented into hospital pharmacy, online provider, and retail pharmacy. The hospital pharmacy segment dominated the market in 2025 due to high distribution of PEGylated drugs for chronic and specialty disease management. Hospitals provide controlled administration, patient monitoring, and access to high-cost PEGylated therapeutics. Institutional purchasing agreements and strong physician recommendations reinforce revenue leadership. Regulatory support for hospital distribution strengthens adoption. High patient trust in hospital-administered therapies boosts market share. Established hospital networks in North America and Europe maintain dominance.
The online provider segment is expected to witness the fastest growth from 2026 to 2033, driven by increasing e-pharmacy adoption and home delivery convenience for chronic disease patients. Online platforms offer competitive pricing, ease of access, and subscription-based models. The segment benefits from digitalization trends and telemedicine integration. Patients in remote regions can access PEGylated drugs without traveling to hospitals. Rising awareness about online pharmacy reliability supports adoption. Partnerships with logistics providers ensure timely delivery and storage compliance.
PEGylated Drugs Market Regional Analysis
- North America dominated the PEGylated drugs market with the largest revenue share of 43.2% in 2025, characterized by advanced healthcare infrastructure, high R&D investment, and a strong presence of leading pharmaceutical companies, with the U.S. experiencing substantial growth driven by innovations in PEGylated interferons and colony-stimulating factors and increasing regulatory approvals
- The region benefits from early adoption of innovative biologics and PEGylated therapeutics, supported by favorable regulatory frameworks and widespread access to healthcare services. Patients and healthcare providers in North America highly value the efficacy, long-acting properties, and improved safety profiles offered by PEGylated drugs, particularly for chronic and rare disease treatments
- High patient awareness, reimbursement coverage, and strong collaborations between pharmaceutical companies and research institutes reinforce North America’s dominance, making it a preferred market for both existing and pipeline PEGylated drugs
U.S. PEGylated Drugs Market Insight
The U.S. PEGylated drugs market captured the largest revenue share of 78% in 2025 within North America, fueled by the high adoption of advanced biologics and the presence of leading pharmaceutical and biotech companies. Patients and healthcare providers increasingly prefer PEGylated therapeutics due to their prolonged half-life, improved safety profiles, and enhanced patient compliance. The market growth is further supported by robust R&D investments, regulatory approvals for new PEGylated drugs, and strong healthcare infrastructure. The rising prevalence of chronic and rare diseases, combined with government initiatives promoting advanced therapies, is accelerating adoption. Furthermore, expanding clinical trial activity and collaborations between biotech firms and research institutes are driving market expansion.
Europe PEGylated Drugs Market Insight
The Europe PEGylated drugs market is projected to expand at a significant CAGR throughout the forecast period, primarily driven by rising demand for biologics and advanced therapeutics across oncology, autoimmune, and rare diseases. Increasing healthcare expenditure, coupled with supportive regulatory frameworks, is fostering the adoption of PEGylated drugs. European patients and providers value the improved efficacy, reduced dosing frequency, and safety of PEGylated therapeutics. The market is experiencing growth across hospital and specialty pharmacy channels, with new PEGylated drugs being incorporated into standard treatment protocols. Ongoing innovation in PEGylation technologies further supports market expansion.
U.K. PEGylated Drugs Market Insight
The U.K. PEGylated drugs market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the rising prevalence of chronic and rare diseases and the increasing adoption of biologics. Patient demand for therapies with improved compliance and reduced side effects is contributing to PEGylated drug uptake. In addition, strong healthcare infrastructure, regulatory support, and reimbursement coverage encourage the use of PEGylated therapeutics. The growing trend of personalized medicine and targeted therapies is also stimulating market growth. Clinical research activity and the availability of advanced PEGylated formulations continue to enhance adoption across both hospital and retail pharmacy channels.
Germany PEGylated Drugs Market Insight
The Germany PEGylated drugs market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of advanced biopharmaceutical therapies and rising demand for safer, long-acting treatments. Germany’s strong healthcare system, focus on R&D, and patient preference for high-efficacy therapies promote PEGylated drug adoption, particularly in oncology and autoimmune diseases. Integration of PEGylated drugs into treatment guidelines supports widespread use. Regulatory support and reimbursement coverage further encourage uptake. The emphasis on innovation and clinical trial activity enhances access to next-generation PEGylated therapeutics.
Asia-Pacific PEGylated Drugs Market Insight
The Asia-Pacific PEGylated drugs market is poised to grow at the fastest CAGR of 23% during the forecast period of 2026 to 2033, driven by rising prevalence of chronic and rare diseases, increasing healthcare expenditure, and rapid adoption of advanced therapies in countries such as China, Japan, and India. Government initiatives promoting healthcare modernization and biologics accessibility are accelerating market adoption. Expanding hospital networks, improved healthcare infrastructure, and growing patient awareness support PEGylated drug uptake. In addition, increasing local manufacturing capabilities in APAC reduce costs and improve availability, driving broader access to PEGylated therapeutics.
Japan PEGylated Drugs Market Insight
The Japan PEGylated drugs market is gaining momentum due to the country’s advanced healthcare system, aging population, and high adoption of biologics. Japanese patients and healthcare providers prioritize therapies with improved safety, efficacy, and patient compliance. The market benefits from strong clinical research activity, government support for innovative therapies, and early adoption of next-generation PEGylated drugs. Integration of PEGylated therapeutics into hospital and specialty pharmacy channels is enhancing treatment accessibility. Rising prevalence of chronic diseases such as hepatitis, cancer, and autoimmune disorders is further driving demand.
India PEGylated Drugs Market Insight
The India PEGylated drugs market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to expanding healthcare access, rising patient awareness, and increasing adoption of biologics. India’s growing middle class, rapid urbanization, and government initiatives for affordable advanced therapies support market growth. PEGylated drugs are becoming increasingly utilized across hospital, retail, and online pharmacy channels. Local manufacturing of PEGylated biologics improves affordability and availability. The rising prevalence of chronic diseases, combined with improvements in healthcare infrastructure, is further accelerating market adoption.
PEGylated Drugs Market Share
The PEGylated Drugs industry is primarily led by well-established companies, including:
- Merck & Co., Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Amgen Inc. (U.S.)
- AstraZeneca (U.K.)
- Novo Nordisk A/S (Denmark)
- Biogen (U.S.)
- BioMarin. (U.S.)
- Coherus BioSciences, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Dr. Reddy’s Laboratories Ltd. (India)
- UCB (Belgium)
- ENZON Pharmaceuticals, Inc. (U.S.)
- Horizon Therapeutics plc (Ireland)
- Leadiant Biosciences S.p.A. (Italy)
- PharmaEssentia Corp. (Taiwan)
- Fresenius Kabi AG (Germany)
- Amneal Pharmaceuticals, Inc. (U.S.)
- Ascendis Pharma A/S (Denmark)
- Apellis Pharmaceuticals, Inc. (U.S.)
- Profacgen, Inc. (U.S.)
What are the Recent Developments in Global PEGylated Drugs Market?
- In December 2025, Chiesi Global Rare Diseases announced Health Canada approval of Elfabrio® (pegunigalsidase alfa) for Fabry disease. This decision allows Elfabrio a long-acting PEGylated enzyme replacement therapy designed to address the underlying enzyme deficiency in adult Fabry disease patients to be marketed in Canada, expanding its global footprint beyond prior approvals in the U.S. and Europe
- In June 2025, the UK’s MHRA approved Dyrupeg, reinforcing European access to a new pegfilgrastim biosimilar. The UK Medicines and Healthcare products Regulatory Agency (MHRA) granted marketing authorization for Dyrupeg, following its earlier EU authorization, enabling its commercial launch in the U.K. market. Dyrupeg is intended to help manage neutropenia associated with cytotoxic chemotherapy, enhancing patient care options while contributing to competitive biosimilar portfolios in the region
- In April 2025, the European Medicines Agency granted marketing authorization to Dyrupeg (pegfilgrastim) for chemotherapy-induced neutropenia in adults. Dyrupeg, a biosimilar form of pegfilgrastim designed to reduce the duration of neutropenia and prevent febrile neutropenia in cancer patients, received EUwide authorization based on its similarity to an established reference medicine
- In May 2023, both the FDA and European Commission approved Elfabrio (pegunigalsidase alfa) for adult Fabry disease patients. Elfabrio received simultaneous regulatory nods in the U.S. and EU, marking one of the most significant PEGylated drug approvals in rare disease care. This dual approval validated the clinical benefits of pegylated enzyme replacement, including prolonged half-life and improved pharmacokinetics, offering a new long-acting therapeutic alternative to traditional treatments for Fabry disease patients
- In May 2023, Amneal Commercialized FYLNETRA™ a pegfilgrastim biosimilar indicated to help decrease febrile neutropenia incidence in cancer patients undergoing chemotherapy marking another competitive PEGylated biologic launch in the U.S. biosimilar market. FYLNETRA’s entry expands patient access to effective, lower-cost supportive oncology therapies, reflecting the broader trend toward biosimilar adoption in PEGylated drug categories
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.