Global Pharmacogenetic Testing Market
Market Size in USD Million
CAGR :
% 
 USD
610.34 Million
USD
1,346.87 Million
2024
2032
USD
610.34 Million
USD
1,346.87 Million
2024
2032
| 2025 –2032 | |
| USD 610.34 Million | |
| USD 1,346.87 Million | |
|
|
|
|
Pharmacogenetic Testing Market Size
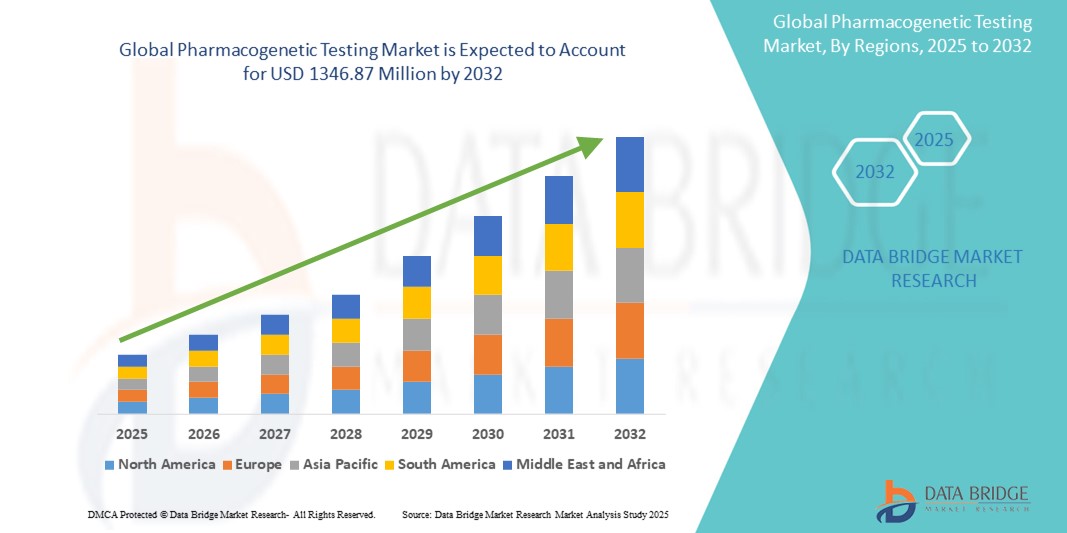
- The global Pharmacogenetic Testing market was valued at USD 610.34 million in 2024 and is expected to reach USD 1346.87 million by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 10.4%, primarily driven by the increasing adoption of personalized medicine,
- This growth is driven by factors such as the advancements in genomic technologies, rising prevalence of chronic diseases, and growing awareness of tailored therapies
Pharmacogenetic Testing Market Analysis
- The consumables segment is anticipated to dominate the market, this dominance is attributed to the increasing demand for customized drugs, which necessitate specialized consumables for pharmacogenetic testing
- This preference is due to its comprehensive ability to analyze genetic variations that influence drug response, providing valuable insights for personalized treatment plans
- Variations in this gene significantly affect the metabolism of several antidepressants, making it a critical target for pharmacogenetic testing in depression treatment
- Hospitals and clinics are anticipated to be the leading end-users. Their significant share is driven by the increasing integration of pharmacogenetic testing into clinical practice to enhance treatment outcomes for patients with psychiatric disorders
- For instance, a study conducted in British Columbia, Canada, demonstrated that pharmacogenomic testing for antidepressants could reduce the time and cost associated with finding the most effective medication for patients.
Report Scope and Pharmacogenetic Testing Market Segmentation
|
Attributes |
Pharmacogenetic Testing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Pharmacogenetic Testing Market Trends
“Increasing Integration of AI and ML in Pharmacogenetic Testing”
- In the pharmacogenetic testing market is the increasing integration of artificial intelligence (AI) and machine learning (ML) into genetic testing platforms. These technologies are being used to analyze vast amounts of genetic data more accurately and efficiently, making pharmacogenetic testing faster and more accessible
- AI and ML algorithms can quickly process large datasets from genomic testing, identifying patterns and correlations that may be missed by traditional analysis methods. This enhances the predictive accuracy of pharmacogenetic tests, which helps in tailoring more effective treatment strategies for patients with psychiatric disorders such as depression and anxiety
- Companies such as Tempus are already using AI-driven platforms to analyze patient data, including genomic sequences, to provide personalized drug recommendations for psychiatric patients. This trend is expected to grow as more AI platforms are developed to support pharmacogenetic testing
- With AI's ability to analyze real-time data, pharmacogenetic testing can be continuously updated to incorporate the latest findings from clinical trials and research studies
- For instance, Illumina’s AI-powered genomic sequencing tools are being integrated into clinical workflows, helping healthcare providers make quicker, data-driven decisions on medication prescriptions for patients
- The use of AI and ML in pharmacogenetic testing has the potential to reduce costs associated with the analysis and interpretation of genetic data
- For Instance, automated data processing reduces the need for manual labor, which is often a major cost factor, making testing more affordable and accessible across various markets
Pharmacogenetic Testing Market Dynamics
Driver
“Increasing Adoption of Personalized Medicine”
- Personalized medicine is gaining traction due to its focus on tailoring treatments to individual genetic profiles, which improves drug efficacy and reduces adverse effects. This is particularly important in psychiatry, where standard treatments may not work for everyone due to genetic variations
- Pharmacogenetic testing helps identify genetic markers that affect drug metabolism and response, such as variations in the CYP2D6 gene, which impacts how patients metabolize antidepressants. By identifying these variations, doctors can prescribe drugs that are more likely to work for the patient with fewer side effects
- Advances in genomic technologies such as next-generation sequencing (NGS) are making pharmacogenetic testing more accessible and affordable, facilitating wider adoption in clinical settings.
- For instance, in the U.S., companies such as Thermo Fisher and Illumina are working to reduce the cost of genetic tests, bringing personalized medicine to more patients
- Mental health conditions such as depression and anxiety, where drug efficacy varies widely, are driving the need for pharmacogenetic testing. Research has shown that genetic testing can help patients with depression find the right antidepressant faster, reducing trial and error in treatment
- For Instance, partnership between Genelex and healthcare providers in the U.S., where their pharmacogenetic testing helps patients with psychiatric disorders find optimal medications based on their genetic profiles
Opportunity
“Expansion in Emerging Markets”
- Emerging markets, particularly in Asia-Pacific and Latin America, are seeing rapid growth in healthcare access, driven by economic development and government initiatives. As a result, there is an increasing need for advanced diagnostic tools such as pharmacogenetic testing
- Countries such as China and India have large populations experiencing a rise in mental health issues, such as depression and bipolar disorder. Pharmacogenetic testing can help address the need for personalized treatment strategies in these regions
- Governments in emerging markets are increasingly investing in healthcare infrastructure, which includes funding for genetic testing technologies.
- With the reduction in testing costs due to technological advancements and increasing public-private partnerships, pharmacogenetic testing is becoming more accessible in emerging markets.
- For instance, Illumina has expanded its genomic sequencing services to countries in Asia, making personalized medicine more affordable
- For Instance, QIAGEN’s expansion into India and China, where they are supporting pharmacogenetic testing as part of the effort to enhance personalized treatment for psychiatric conditions, thereby tapping into an underserved market
Restraint/Challenge
“High Cost of Testing and Infrastructure Limitations”
- One of the most significant challenges to the widespread adoption of pharmacogenetic testing is the high cost of genetic testing
- For Instance, the cost of whole genome sequencing can still be prohibitively expensive for many patients, especially in developing countries
- Many emerging markets lack the necessary infrastructure, such as specialized laboratories and trained personnel, to carry out pharmacogenetic tests. This limits the availability of testing in rural or low-resource areas, where it is needed most to manage complex psychiatric disorders
- In many countries, insurance companies do not fully reimburse pharmacogenetic testing, leaving patients to pay out of pocket. This is especially problematic in countries with underfunded healthcare systems.
- For instance, despite the growing interest in pharmacogenetic testing in the U.S., many insurance companies still do not cover the costs for psychiatric conditions such as depression
- Even where genetic tests are available, interpreting the results and integrating them into treatment plans is a complex process. This requires highly trained professionals, and the lack of such experts in some regions can hinder the effective use of pharmacogenetic testing
- For Instance, Sonic Healthcare in Australia, which has encountered limitations in adopting pharmacogenetic testing across all its facilities due to high costs and the need for additional infrastructure and personnel to handle the testing process
Pharmacogenetic Testing Market Scope
The market is segmented on the basis type, product, test type, gene type, patient type, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Product |
|
|
By Test Type |
|
|
By Gene Type
|
|
|
By Patient Type |
|
|
By End-User
|
|
|
By Distribution Channel |
|
Pharmacogenetic Testing Market Regional Analysis
“North America is the Dominant Region in the Pharmacogenetic Testing Market”
- North America is projected to maintain its dominance in the pharmacogenetic testing market for psychiatry and depression
- The region boasts advanced healthcare infrastructure and substantial investments in research and development, facilitating the integration of pharmacogenetic testing into clinical practices
- Supportive regulatory frameworks and reimbursement policies have been instrumental in promoting the adoption of personalized medicine approaches, including pharmacogenetic testing
- Hospitals and clinics in North America are increasingly incorporating pharmacogenetic testing to tailor psychiatric treatments, enhancing patient outcomes and reducing adverse drug reactions
- Given these factors, North America is expected to continue leading the global market in the coming years
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is projected to experience the highest growth rate in the pharmacogenetic testing market for psychiatry and depression during the forecast period
- Countries such as China and India are witnessing rapid economic growth, leading to improved healthcare access and increased demand for advanced diagnostic services
- The rising geriatric population in the region is contributing to a higher prevalence of psychiatric disorders, thereby driving the need for personalized treatment options
- Collaborations between local diagnostic players and global leaders, along with increased investments in healthcare infrastructure, are accelerating the adoption of pharmacogenetic testing
- Supportive government policies and reimbursement schemes are further facilitating the integration of pharmacogenetic testing into psychiatric care across the region
Pharmacogenetic Testing Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Thermo Fisher Scientific Inc. (U.S.)
- Illumina Inc. (U.S.)
- Myriad Genetics Inc. (U.S.)
- Sonic Healthcare Limited (Australia)
- QIAGEN (Germany)
- AB-BIOTICS S.A. (Spain)
- BiogeniQ Inc. (India)
- Castle Bioscience Inc. (U.S.)
- Coriell Life Sciences (U.S.)
- Dynamic DNA Laboratories (U.S.)
- Eurofins Scientific (Luxembourg)
- Genelex (U.S.)
- Genewiz (U.S.)
- Genomind Inc. (U.S.)
- GenXys (Canada)
- HealthSpek (U.S.)
- HudsonAlpha (U.S.)
- MD Labs (U.S.)
- ONEOME LLC (U.S.)
- PacBio (U.S.)
Latest Developments in Global Pharmacogenetic Testing Market
- In February 2024, Myriad Genetics, Inc., a pioneer in genetic testing and precision medicine, has acquired select assets from Intermountain Health's Precision Genomics (IPG) laboratory. This includes the Precise Tumor Test, the Precise Liquid Test, and IPG's CLIA-certified laboratory in St. George, Utah. This acquisition allows Myriad to enhance its tumor profiling offerings and expand its oncology portfolio, which includes hereditary cancer and companion diagnostic testing options
- In February 2024, QIAGEN announced that it has been recognized for its sustainability efforts by My Green Lab, a non-profit organization focused on promoting sustainability in scientific research. This will enhance QIAGEN's reputation for sustainability, potentially attracting environmentally conscious customers and partners
- In November 2023, QIAGEN and DNA Labs International collaborated to solve two decades-old cold cases using QIAGEN's ForenSeq Kintelligence System for forensic genetic genealogy, in conjunction with the GEDmatch PRO database. This highlights the effectiveness and increasing adoption of this approach to human identification. This will result in the effectiveness of QIAGEN's forensic genetic genealogy solutions, potentially boosting their adoption and sales in the forensic market
- In June 2022, Castle Biosciences, Inc, a company improving health through innovative tests that guide patient care, today announced it has signed a definitive agreement to acquire AltheaDx, Inc. This acquisition would enable to potentially develop a mental health franchise, starting with a test that currently receives Medicare reimbursement for depression
- In May 2022, Coriell Life Sciences won Second MedTech Breakthrough Award for Genomics Innovation which leads the way in unlocking the power of pharmacogenomics to improve healthcare. the organization’s Corigen Medication Safety Program has been named “Best Overall Genomics Solution” in the 2022 MedTech Breakthrough Awards. The award will give the testing more credibility among the users
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.