Global Phenylketonuria Market
Market Size in USD Million
CAGR :
% 
 USD
1.27 Million
USD
2.44 Million
2024
2032
USD
1.27 Million
USD
2.44 Million
2024
2032
| 2025 –2032 | |
| USD 1.27 Million | |
| USD 2.44 Million | |
|
|
|
Phenylketonuria Market Analysis
The phenylketonuria market is experiencing steady growth, driven by an increasing prevalence of this rare genetic disorder and growing awareness among healthcare professionals and the public. Phenylketonuria is a metabolic disorder where the body cannot break down an amino acid called phenylalanine, leading to potential brain damage if left untreated. The market is characterized by advancements in diagnostic technologies, as well as treatments that aim to manage the condition.
As the global population becomes more aware of the importance of early detection and proper management of phenylketonuria, the demand for diagnostic tools and therapeutic solutions has risen. There is a notable focus on improving the quality of life for individuals affected by phenylketonuria, with a particular emphasis on dietary management and enzyme replacement therapies.
Pharmaceutical companies are actively researching novel treatments to address the metabolic deficiencies caused by the disorder. This includes developing therapies that aim to reduce the accumulation of phenylalanine or provide alternative metabolic pathways.
The market is also witnessing an increase in government initiatives and funding for research into rare diseases such as phenylketonuria, which is fuelling the development of new diagnostic and treatment options. With increasing awareness, improved healthcare access, and ongoing research efforts, the phenylketonuria market is expected to continue expanding in the coming years.
Phenylketonuria Market Size
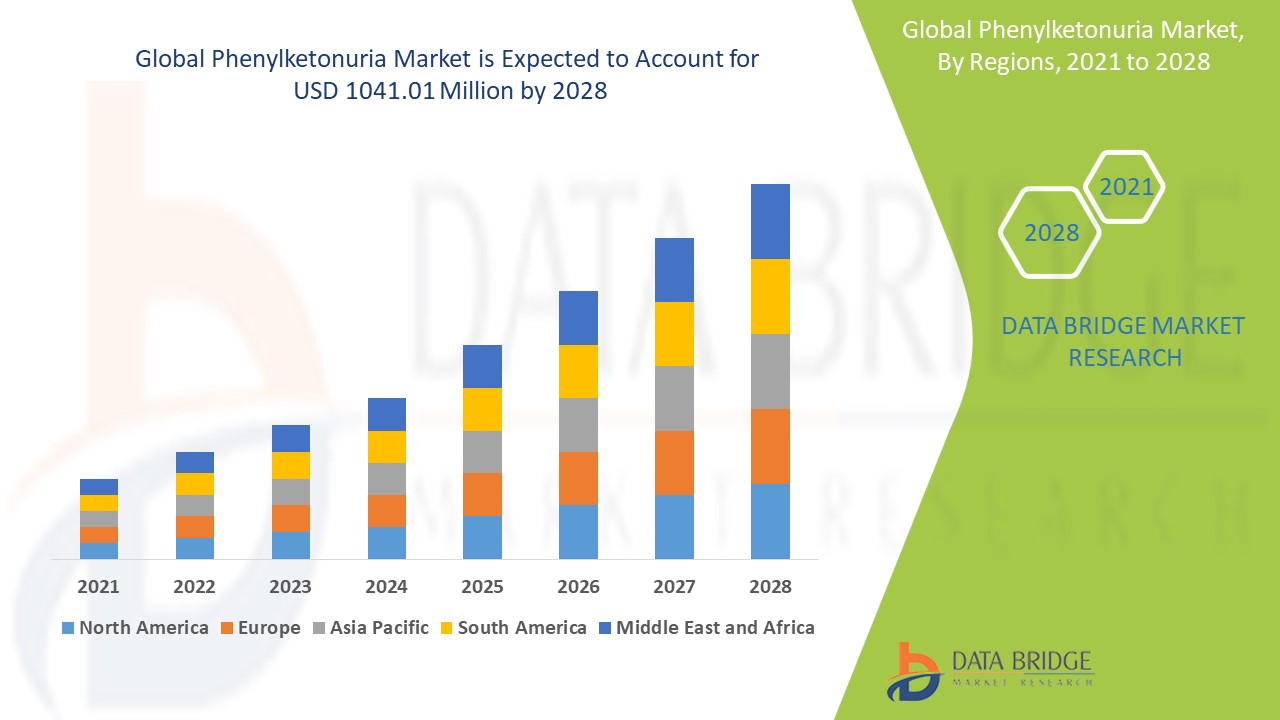
The phenylketonuria market size was valued at USD 1.27 billion in 2024 and is projected to reach USD 2.44 million by 2032, with a CAGR of 8.51% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Phenylketonuria Market Trends
“Growing focus on personalized treatments”
The growing focus on personalized treatments in the phenylketonuria market is a trend that is transforming how the condition is managed. Personalized treatments refer to tailored therapies that are designed to meet the specific needs of individual patients based on their genetic makeup, lifestyle, and the severity of their condition. For phenylketonuria, this approach is becoming increasingly important because the disorder can vary significantly from one patient to another.
With personalized treatments, healthcare providers can move away from a one-size-fits-all strategy and instead offer more targeted interventions. This could involve customizing drug regimens, dietary plans, or even gene therapies based on the unique genetic characteristics of the individual. For instances, some patients may respond better to specific enzyme replacement therapies or medications that help manage phenylalanine levels in the blood, while others may benefit from dietary supplements that are specifically designed for their metabolic needs.
The move towards personalized treatments is also being driven by advances in genetic testing, which enable a deeper understanding of how different mutations affect the metabolism of phenylalanine. With more precise diagnostic tools, healthcare professionals are better equipped to develop individualized treatment plans that improve patient outcomes and quality of life.
This trend reflects a broader shift toward precision medicine, where treatments are increasingly designed with the patient at the center, ensuring more effective and efficient care in the management of phenylketonuria.
Report Scope Phenylketonuria Market Segmentation
|
Attributes |
Phenylketonuria Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
DAIICHI SANKYO COMPANY, LIMITED (Japan), Ajinomoto Cambrooke, Inc. (U.S.), American Gene Technologies (U.S.), Ultragenyx Pharmaceutical Inc. (U.S.), Nutricia (Netherlands), Orphanet (France), Abbott (U.S.), Travere Therapeutics, Inc. (U.S.), Synlogic (U.S.), SOM BIOTECH (Spain), Codexis, Inc. (U.S.), BioMarin (U.S.), Phaxiam (France), Mead Johnson & Company, LLC. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Phenylketonuria Market Definition
Phenylketonuria is a rare genetic disorder in which the body is unable to break down an amino acid called phenylalanine. This happens because of a deficiency in an enzyme called phenylalanine hydroxylase, which normally converts phenylalanine into another substance that the body needs. When phenylalanine builds up in the blood, it can lead to serious health problems, especially brain damage, as it is toxic to the nervous system.
Phenylketonuria Market Dynamics
Drivers
- Rising Prevalence of Phenylketonuria
The increasing prevalence of phenylketonuria is a significant driver for the market. This rare genetic disorder affects new-borns worldwide, with many cases going undiagnosed until later in life. Early screening programs, particularly in new-borns, have been instrumental in identifying affected individuals, increasing the overall number of diagnosed cases. As awareness about the disorder grows and screening programs become more widespread, there is an escalating demand for both diagnostic tools and treatment options. The growing awareness about the importance of early detection is helping to drive the phenylketonuria market forward, particularly in countries where new-born screening programs are being expanded.
- Advancements in Treatment and Therapy Options
The phenylketonuria market is also driven by ongoing advancements in treatment and therapeutic options. Traditionally, the condition has been managed through a strict low-protein diet, but recent innovations, including enzyme replacement therapies, gene therapies, and medications such as sapropterin, offer new hope for improved patient outcomes. These treatments aim to address the underlying metabolic issue in more effective ways, reducing the reliance on dietary restrictions. As these treatment options evolve and more options become available, the market is expanding, with pharmaceutical companies investing in the development of novel therapies to provide better long-term solutions for individuals living with phenylketonuria.
Opportunities
- Growth in Gene Therapy Research
One of the most promising opportunities in the phenylketonuria market is the continued research and development of gene therapies. With advancements in gene-editing technologies such as CRISPR, scientists are now exploring ways to correct the genetic mutation that causes phenylketonuria at its root. Gene therapy could potentially provide a long-term solution by enabling the body to produce the necessary enzyme to break down phenylalanine, addressing the metabolic defect directly. If successful, gene therapy could significantly reduce the dependence on lifelong dietary restrictions and therapies, offering a more permanent treatment option for patients. This potential breakthrough presents a lucrative opportunity for pharmaceutical companies and researchers in the market.
- Expansion of Personalized Medicine
As the field of personalized medicine grows, the phenylketonuria market is poised to benefit from treatments tailored to individual patients. Personalized treatments involve designing therapies based on a patient's unique genetic profile, lifestyle, and disease severity. This could lead to more effective treatments and better management of phenylketonuria, as not all patients react to therapies in the same way. Precision medicine could also open up new avenues for improving the patient experience, enhancing treatment adherence, and minimizing adverse effects. The opportunity to integrate genetic testing and customized therapeutic options makes personalized medicine a key area of growth for the phenylketonuria market, with significant investment potential.
Restraints/Challenges
- High Treatment Costs and Financial Barriers
A key restraint in the phenylketonuria market is the high cost associated with treatment and management. Phenylketonuria is a lifelong condition, and patients often need to follow strict dietary plans and take medications or supplements to manage the disorder. In addition, enzyme replacement therapies and genetic treatments are often expensive and may not be accessible to all patients, particularly in lower-income regions. The ongoing costs of care, coupled with the price of new, cutting-edge therapies, can be a significant burden on both patients and healthcare systems. These financial barriers may limit the adoption of advanced treatments, hindering the overall growth of the market.
- Limited awareness in low-resource regions
A significant challenge facing the phenylketonuria market is the limited awareness of the condition in certain regions. While new-born screening is common in many developed countries, it is still not universally implemented worldwide, especially in low- and middle-income countries. As a result, many children with phenylketonuria go undiagnosed until later in life, which can lead to irreversible developmental and neurological damage. The lack of awareness and resources in these regions creates a significant barrier to timely diagnosis and treatment, ultimately limiting the effectiveness of market interventions. Raising awareness and improving screening programs in underserved areas remains a critical challenge for the phenylketonuria market.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Phenylketonuria Market Scope
The market is segmented on the basis of types, test, treatment, and application growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Types
- Classic PKU
- Mild PKU
- Benign PKU
Test
- Diagnostic Test
- Screening Test
Treatment
- Drug Therapy
- Dietary Therapy
- Gene Therapy
Application
- Household
- Hospitals
- Others
Phenylketonuria Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, types, test, treatment, and application as referenced above.
The countries covered in the market report are U.S., Canada, Mexico in North America, Germany, Sweden, Poland, Denmark, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe in Europe, Japan, China, India, South Korea, New Zealand, Vietnam, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in Asia-Pacific (APAC), Brazil, Argentina, Rest of South America as a part of South America, U.A.E, Saudi Arabia, Oman, Qatar, Kuwait, South Africa, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA).
North America dominates the phenylketonuria market, thanks to its well-established healthcare infrastructure, comprehensive new-born screening programs, and high prevalence of the condition. The region has robust systems for early detection, allowing for prompt diagnosis and management of phenylketonuria. This, coupled with advanced healthcare technology and strong awareness campaigns, ensures that affected individuals receive timely and effective treatment. In addition, the presence of key pharmaceutical companies and a high level of research and development further strengthen North America's leading position in the market.
Europe is the fastest-growing region in the phenylketonuria market, with significant efforts to improve awareness and treatment options for the disorder. Many European countries have implemented mandatory new-born screening programs, which have contributed to an increase in early diagnoses and better management of phenylketonuria. The rising demand for specialized dietary products and enzyme replacement therapies has driven market growth, as more individuals seek advanced treatments to manage the condition. Moreover, the expansion of healthcare access and innovations in medical technology are fuelling the rapid growth of the market in Europe, making it a key player in the global phenylketonuria landscape.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Phenylketonuria Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Phenylketonuria Market Leaders Operating in the Market Are:
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- Ajinomoto Cambrooke, Inc. (U.S.)
- American Gene Technologies (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Nutricia (Netherlands)
- Orphanet (France)
- Abbott (U.S.)
- Travere Therapeutics, Inc (U.S.)
- Synlogic (U.S.)
- SOM BIOTECH (Spain)
- Codexis, Inc. (U.S.)
- BioMarin (U.S.)
- Phaxiam (France)
- Mead Johnson & Company, LLC. (U.S.)
Latest Developments in Phenylketonuria Market
- In January 2024, Jnana Therapeutics announced positive clinical proof of concept for JNT-517, a potential first-in-class oral treatment for phenylketonuria (PKU). This treatment is designed to address the underlying metabolic deficiency in PKU by enabling the body to break down phenylalanine, the amino acid that accumulates in patients with the disorder. The successful study results highlight JNT-517’s potential to offer a new therapeutic option for PKU patients, providing an alternative to strict dietary management. If approved, this oral treatment could improve the quality of life for individuals living with PKU by reducing reliance on dietary restrictions
- In May 2024, PTC Therapeutics announced that the European Medicines Agency (EMA) will review its Marketing Authorization Application (MAA) for Sepiapterin, a treatment for phenylketonuria (PKU). Sepiapterin is designed to reduce the levels of phenylalanine in PKU patients by enhancing the body’s ability to produce the necessary enzyme to metabolize it. If approved, this therapy would provide a novel option for PKU patients, offering a potential treatment to complement dietary restrictions and improve overall management of the condition. The EMA’s review is a critical step toward bringing this innovative treatment to the market
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.